Brainstem catecholaminergic neurons induce torpor during fasting by orchestrating cardiovascular and thermoregulation changes
- PMID: 40593766
- PMCID: PMC12214849
- DOI: 10.1038/s41467-025-61179-1
Brainstem catecholaminergic neurons induce torpor during fasting by orchestrating cardiovascular and thermoregulation changes
Abstract
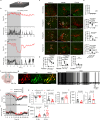
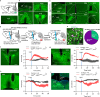
Torpor, an adaptive hypometabolic state in response to fasting, is characterized by pronounced reductions in body temperature, heart rate, and thermogenesis. However, how the brain orchestrates these physiological changes to induce torpor and the relationships among them remain elusive. Inhibiting catecholaminergic (CA) neurons in the ventrolateral medulla (VLM) significantly impairs torpor in mice, while their activation reduces body temperature, heart rate, energy expenditure, physical activity, and thermogenesis. Importantly, the heart rate decline precedes body temperature reduction, resembling patterns observed in natural torpid animals. Moreover, a likely causal relationship exists between heart rate reduction and body temperature decline. VLM-CA neurons may regulate heart rate and thermogenesis through projections to the dorsal motor vagal nucleus and medial preoptic area, respectively. Additionally, these neurons are conserved in Daurian ground squirrels and become active before hibernation, indicating their potential role in hibernation. Here, we find that VLM-CA neurons play important roles in fasting-induced torpor.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
Metabolic hormone FGF21 is induced in ground squirrels during hibernation but its overexpression is not sufficient to cause torpor.PLoS One. 2013;8(1):e53574. doi: 10.1371/journal.pone.0053574. Epub 2013 Jan 2. PLoS One. 2013. PMID: 23301087 Free PMC article.
-
A torpor-like state in mice slows blood epigenetic aging and prolongs healthspan.Nat Aging. 2025 Mar;5(3):437-449. doi: 10.1038/s43587-025-00830-4. Epub 2025 Mar 7. Nat Aging. 2025. PMID: 40055478 Free PMC article.
-
Estrogen-sensitive medial preoptic area neurons coordinate torpor in mice.Nat Commun. 2020 Dec 11;11(1):6378. doi: 10.1038/s41467-020-20050-1. Nat Commun. 2020. PMID: 33311503 Free PMC article.
-
The Art of Chilling Out: How Neurons Regulate Torpor.Bioessays. 2025 Feb;47(2):e202400190. doi: 10.1002/bies.202400190. Epub 2024 Nov 26. Bioessays. 2025. PMID: 39600072 Free PMC article. Review.
-
Neural Signaling Metabolites May Modulate Energy Use in Hibernation.Neurochem Res. 2017 Jan;42(1):141-150. doi: 10.1007/s11064-016-2109-4. Epub 2016 Nov 23. Neurochem Res. 2017. PMID: 27878659 Free PMC article. Review.
References
-
- Overton, J. M. & Williams, T. D. Behavioral and physiologic responses to caloric restriction in mice. Physiol. Behav.81, 749–754 (2004). - PubMed
-
- Zimmer, M. B. & Milsom, W. K. Effects of changing ambient temperature on metabolic, heart, and ventilation rates during steady state hibernation in golden-mantled ground squirrels (Spermophilus lateralis). Physiol. Biochem. Zool.74, 714–723 (2001). - PubMed
-
- Cerri, M., Hitrec, T., Luppi, M. & Amici, R. Be cool to be far: exploiting hibernation for space exploration. Neurosci. Biobehav. Rev.128, 218–232 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

