ACSS2 protects against alcohol-induced hepatocyte ferroptosis through regulation of hepcidin expression
- PMID: 40593779
- PMCID: PMC12215787
- DOI: 10.1038/s41467-025-61067-8
ACSS2 protects against alcohol-induced hepatocyte ferroptosis through regulation of hepcidin expression
Abstract
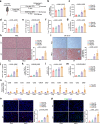
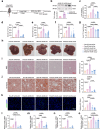
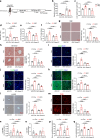
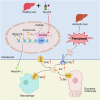
Acetate is the end product of alcohol metabolism. Acyl-CoA synthetase short-chain family member 2 (ACSS2) converts acetate to acetyl-CoA, involving metabolic pathways and epigenetic regulation. However, the function of ACSS2-mediated epigenetic control in alcoholic liver disease (ALD) remains incompletely understood. We demonstrate that alcohol downregulates hepatic ACSS2, causing acetate accumulation in the liver and serum. This disrupts iron metabolism and hepatic ferroptosis, triggering liver injury and inflammation. Mechanistically, ACSS2 binds CREB binding protein (CBP) to mediate histone acetylation and regulate hepcidin antimicrobial peptide 1/2 (HAMP1/2) transcription. ACSS2 deficiency downregulates HAMP1/2, causing systemic iron dyshomeostasis and ferroptosis, which is restored by overexpression of HAMP1/2. Iron chelators or ferroptosis inhibitors attenuates alcohol-induced liver injury in ACSS2-deficient mice. Our study uncovers the epigenetic mechanisms of ACSS2-mediated ferroptosis and its role in ALD progression.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Devarbhavi, H. et al. Global burden of liver disease: 2023 update. J. Hepatol.79, 516–537 (2023). - PubMed
-
- Seitz, H. K. et al. Alcoholic liver disease. Nat. Rev. Dis. Prim.4, 16 (2018). - PubMed
-
- Mascord, D., Smith, J., Starmer, G. A. & Whitfield, J. B. Effects of increasing the rate of alcohol metabolism on plasma acetate concentration. Alcohol Alcohol27, 25–28 (1992). - PubMed
MeSH terms
Substances
Grants and funding
- U22A20272/National Natural Science Foundation of China (National Science Foundation of China)
- 82173807/National Natural Science Foundation of China (National Science Foundation of China)
- 82304503/National Natural Science Foundation of China (National Science Foundation of China)
- 82370813/National Natural Science Foundation of China (National Science Foundation of China)
- 82300516/National Science Foundation of China | Young Scientists Fund
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

