Regenerating aged bone marrow via a nitric oxide nanopump
- PMID: 40593809
- PMCID: PMC12219323
- DOI: 10.1038/s41467-025-61256-5
Regenerating aged bone marrow via a nitric oxide nanopump
Abstract
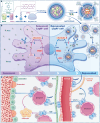
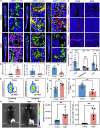
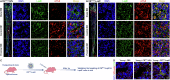
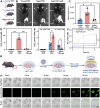
Resident leptin-receptor-expressing (LepR+) cells senescence in the aged bone marrow impairs the regenerative capacity of osteo- and other lineages of cells. In this study, a LepR+ cell-targeting nitric oxide (NO) nanopump with in vivo self-controlled turn-on ability was constructed to rejuvenate the LepR+ cells in the aged bone marrow. The nanopump co-entrapped hydrophobic chemiluminescence substrate and NO donor into the matrix of amphiphilic polymer through a nanoprecipitation process. The chemiluminescence substrate in the NO nanopump automatically reacts with the accumulated H2O2 in the aged bone marrow and then directly transfers the chemical energy to the NO donor to induce in situ NO release. The NO produced in situ within the aged bone marrow triggered the regeneration of the osteoblastic and the other niches in vivo through activating the glycolysis signaling in the senescent LepR+ cells. Conclusively, the constructed NO nanopump is a promising tool to counter aging-induced bone marrow disorders.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interest.
Figures



Similar articles
-
Nitric oxide donors for cervical ripening and induction of labour.Cochrane Database Syst Rev. 2016 Dec 5;12(12):CD006901. doi: 10.1002/14651858.CD006901.pub3. Cochrane Database Syst Rev. 2016. PMID: 27918616 Free PMC article.
-
The potential role of nitrate, a nitric oxide donor, in the prevention and treatment of diabetic osteoporosis.Front Endocrinol (Lausanne). 2025 Jun 12;16:1480838. doi: 10.3389/fendo.2025.1480838. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40575264 Free PMC article. Review.
-
Leptin receptor downregulation in the carotid body treats obesity-induced hypertension.J Neurophysiol. 2025 Mar 1;133(3):892-903. doi: 10.1152/jn.00133.2024. Epub 2025 Feb 4. J Neurophysiol. 2025. PMID: 39903168 Free PMC article.
-
Interventions affecting the nitric oxide pathway versus placebo or no therapy for fetal growth restriction in pregnancy.Cochrane Database Syst Rev. 2023 Jul 10;7(7):CD014498. doi: 10.1002/14651858.CD014498. Cochrane Database Syst Rev. 2023. PMID: 37428872 Free PMC article.
-
Nitric oxide donors for cervical ripening and induction of labour.Cochrane Database Syst Rev. 2011 Jun 15;(6):CD006901. doi: 10.1002/14651858.CD006901.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2016 Dec 05;12:CD006901. doi: 10.1002/14651858.CD006901.pub3. PMID: 21678363 Updated.
References
-
- Yue, R., Zhou, B. O., Shimada, I. S., Zhao, Z. & Morrison, S. J. Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell18, 782–796 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

