Microanatomy related biocidal activity at cellular resolution and bone reconstruction potential of PEG EGaIn nanocapsules
- PMID: 40593811
- PMCID: PMC12216455
- DOI: 10.1038/s41522-025-00724-8
Microanatomy related biocidal activity at cellular resolution and bone reconstruction potential of PEG EGaIn nanocapsules
Abstract
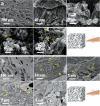
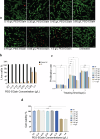
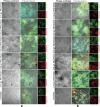
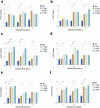
Critical bone defects, exacerbated by infections, pose significant challenges to bone healing and homeostasis, necessitating the development of dual-functional biomimetics that combine anti-infective and reparative capabilities. The EGaIn holds promise across various disciplines, though its interactions with microbial cells require further elucidation. This investigation evaluates the antimicrobial efficacy of PEG-EGaIn nanocapsules against a spectrum of bacterial, employing electron microscopy. Constructs containing 1.5% PEG-EGaIn hinder biofilm-producing bacteria, while 3% concentrations amplify the biocidal effect. Furthermore, the nanocapsules promoted osteogenic differentiation rBMSCs, evidenced by enhanced mineralization and upregulation of key osteogenic genes. In addressing large bone defects, PEG-EGaIn-Col-Ap-lamellar and ethanolic-mediated Col-Ap-lamellar constructs serve as potential solutions for bone resorption mitigation and osteo-angiogenesis. Bone-remodeling were validated through μ-CT and histomorphometry confirming no evidence of chronic inflammation or fibrosis. In this study, PEG-EGaIn nanocapsules emerge as potent biocide and bone repair, underscoring their potential in combating antibiotic resistance and enhancing bone healing.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Mineralized osteoblast-derived exosomes and 3D-printed ceramic-based scaffolds for enhanced bone healing: A preclinical exploration.Acta Biomater. 2025 Jun 15;200:686-702. doi: 10.1016/j.actbio.2025.05.051. Epub 2025 May 21. Acta Biomater. 2025. PMID: 40409510
-
Construction and Characterization of Polyethylene Glycol/Sodium Alginate Hydrogel Loaded With Zirconia Nanoparticles: Potential Antibacterial and Antibiofilm Agent to Inhibit Dental Caries In Vitro and In Vivo.Microsc Res Tech. 2025 Aug;88(8):2268-2284. doi: 10.1002/jemt.24860. Epub 2025 Mar 21. Microsc Res Tech. 2025. PMID: 40114660
-
Glycinamide Facilitates Nanocomplex Formation and Functions Synergistically with Bone Morphogenetic Protein 2 to Promote Osteoblast Differentiation In Vitro and Bone Regeneration in a Mouse Calvarial Defect Model.Tissue Eng Regen Med. 2024 Oct;21(7):1093-1107. doi: 10.1007/s13770-024-00657-x. Epub 2024 Jul 2. Tissue Eng Regen Med. 2024. PMID: 38955905 Free PMC article.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
A systematic review on natural products with antimicrobial potential against WHO's priority pathogens.Eur J Med Res. 2025 Jul 1;30(1):525. doi: 10.1186/s40001-025-02717-x. Eur J Med Res. 2025. PMID: 40597250 Free PMC article.
References
-
- De Long, W. G. Jr. et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J. Bone Jt. Surg. Am.89, 649–658 (2007). - PubMed
-
- Roope, L. S. J. et al. The challenge of antimicrobial resistance: what economics can contribute. Science364, eaau4679 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

