Self-reinforced piezoelectric chip for scaffold-free repair of critical-sized bone defects
- PMID: 40593819
- PMCID: PMC12216264
- DOI: 10.1038/s41467-025-61243-w
Self-reinforced piezoelectric chip for scaffold-free repair of critical-sized bone defects
Abstract
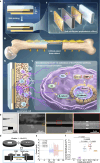
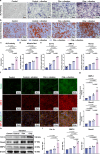
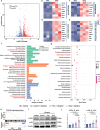
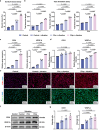
The use of piezoelectric materials to treat critical-sized bone defects typically requires additional stimulation to generate their piezoelectric properties and the implantation of scaffolds to promote bone repair. Here we present a self-reinforced piezoelectric chip and demonstrate its efficacy in the treatment of critical-sized bone defects. Specifically, the chip is comprised of the third-generation semiconductor aluminum nitride (AlN) as a piezoelectric layer, molybdenum (Mo) electrodes, and a silicon substrate with an optimized internal cavity structure. All these components are confirmed to be non-cytotoxic. This design enables the chip to provide self-sustained and long-term electrical signals in response to physiological vibrations. After being implanted into a rabbit critical-sized femoral defect model, the chip creates a localized bioelectric microenvironment, thereby promoting vascularized bone repair within 4 weeks without using any scaffolds and additional tools. Moreover, the chip can be fixed onto the clinically used orthopedic plate system, representing a universal plug-and-play strategy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Does Augmenting Irradiated Autografts With Free Vascularized Fibula Graft in Patients With Bone Loss From a Malignant Tumor Achieve Union, Function, and Complication Rate Comparably to Patients Without Bone Loss and Augmentation When Reconstructing Intercalary Resections in the Lower Extremity?Clin Orthop Relat Res. 2025 Jun 26. doi: 10.1097/CORR.0000000000003599. Online ahead of print. Clin Orthop Relat Res. 2025. PMID: 40569278
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Guided tissue regeneration for periodontal infra-bony defects.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD001724. doi: 10.1002/14651858.CD001724.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2019 May 29;5:CD001724. doi: 10.1002/14651858.CD001724.pub3. PMID: 16625546 Updated.
-
Mineralized osteoblast-derived exosomes and 3D-printed ceramic-based scaffolds for enhanced bone healing: A preclinical exploration.Acta Biomater. 2025 Jun 15;200:686-702. doi: 10.1016/j.actbio.2025.05.051. Epub 2025 May 21. Acta Biomater. 2025. PMID: 40409510
-
Incentives for preventing smoking in children and adolescents.Cochrane Database Syst Rev. 2017 Jun 6;6(6):CD008645. doi: 10.1002/14651858.CD008645.pub3. Cochrane Database Syst Rev. 2017. PMID: 28585288 Free PMC article.
References
-
- Schemitsch, E. H. Size matters: Defining critical in bone defect size!. J. Orthop. Trauma31, S20–S22 (2017). - PubMed
-
- Yuan, X. et al. Recent advances in 3D printing of smart scaffolds for bone tissue engineering and regeneration. Adv. Mater.36, e2403641 (2024). - PubMed
-
- Nain, A. et al. Progress in the development of piezoelectric biomaterials for tissue remodeling. Biomaterials307, 122528 (2024). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

