Paraventricular hypothalamic input to anterior cingulate cortex controls food preferences in chronic visceral pain mice
- PMID: 40593837
- PMCID: PMC12217335
- DOI: 10.1038/s41467-025-61178-2
Paraventricular hypothalamic input to anterior cingulate cortex controls food preferences in chronic visceral pain mice
Abstract
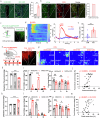
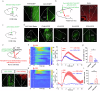
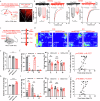
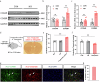
Chronic visceral pain is frequently accompanied by changes in food preference. The paraventricular hypothalamus (PVH) and the anterior cingulate cortex (ACC) are well-known regions involved in pain processing and food intake. However, the underlying neural circuitry mechanisms remain unclear. Here, we showed that a circuit from cholecystokinin neurons in the PVH (PVHCCK) projecting to glutamatergic neurons in the ACC (ACCGlu) to regulate food preference in male mice with chronic visceral pain induced by neonatal colonic inflammation (NCI). The mice with chronic visceral pain preferred for sucrose when compared with control mice. Chemogenetic inhibition of the PVHCCK to ACCGlu circuit reduced chronic visceral pain and led to food preference switched from sucrose to intralipid, which was reversed by an injection of an agonist of CCKBRs in the ACC. Chemogenetic activation of PVHCCK to ACCGlu circuit increased visceral pain and resulted in food preference switched from intralipid to sucrose, which was reversed by an injection of an antagonist of cholecystokinin receptors (CCKBRs) in the ACC. Our study indicates that the PVHCCK to ACCGlu circuit encodes changes in food preference during chronic visceral pain. Intervention targeting this neural circuitry might be a potential therapeutic strategy for patients with chronic visceral pain.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The author(s) declare no competing interests.
Figures



References
-
- Elsenbruch, S., Häuser, W. & Jänig, W. Visceral pain. Schmerz (Berl., Ger.)29, 496–502 (2015). - PubMed
-
- Zamyad, M., Abbasnejad, M., Esmaeili-Mahani, S., Sheibani, V. & Raoof, M. Pain influences food preference and food-related memory by activating the basolateral amygdala in rats. Exp. Brain Res.239, 79–93 (2021). - PubMed
-
- Verbanac, D., Maleš, Ž & Barišić, K. Nutrition - facts and myths. Acta Pharm. (Zagreb, Croat.)69, 497–510 (2019). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

