The function of PCSK9 in doxorubicin-induced cardiotoxicity and its underlying mechanism
- PMID: 40593844
- PMCID: PMC12215775
- DOI: 10.1038/s41598-025-03419-4
The function of PCSK9 in doxorubicin-induced cardiotoxicity and its underlying mechanism
Abstract
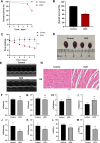
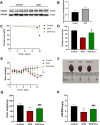
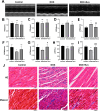
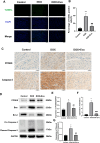
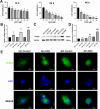
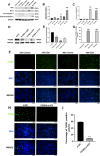
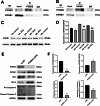
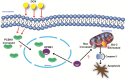
Doxorubicin (DOX) is an anthracycline class of chemotherapy drug, the application of which is limited due to its cardiotoxic effects. Recombinant Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9), is a serine protease pivotal in lipid metabolism and has a profound correlation with the onset of cardiovascular diseases. This study uncovers a connection between PCSK9 and DOX-induced cardiotoxicity (DIC). This research found that injection of DOX in mice caused cardiac toxicity. DOX treatment up-regulated the expression of PCSK9 protein in myocardial tissue. Evolocumab (PCSK9 inhibitors) improved cardiac function, myocardial injury, and fibrosis in DOX-treated mice, indicating a protective effect against DIC. The mechanism involved modulation of cardiomyocyte apoptosis and regulation of apoptosis-related proteins, including Bax/Bcl-2 ratio and Cleaved Caspase-3/Pro Caspase-3 ratio. DOX exhibited concentration- and time-dependent cytotoxic effects on H9C2 cardiomyocytes, promoting apoptosis. PCSK9 nuclear aggregation occurred in H9C2 cardiomyocytes after DOX treatment, and PCSK9 interacted with the Importin subunit beta-1 (KPNB1) protein. Interference with PCSK9 up-regulated KPNB1 expression, affecting apoptosis-related proteins and improving DOX-induced H9C2 cardiomyocyte apoptosis. In short, the elucidation of this mechanism is helpful involve that PCSK9 inhibitor may be a potential drug for improving DIC.
Keywords: Apoptosis; Cardiotoxicity; Doxorubicin; KPNB1; PCSK9.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable.
Figures
References
-
- Deo, S. V., Al-Kindi, S. G. & Oliveira, G. H. Management of advanced heart failure due to cancer therapy: the present role of mechanical circulatory support and cardiac transplantation. Curr. Treat. Opt. Cardiovasc. Med.17, 388. 10.1007/s11936-015-0388-8 (2015). - PubMed
-
- Renu, K., Abilash, V. G. & Arunachalam, S. Molecular mechanism of doxorubicin-induced cardiomyopathy—an update. Eur. J. Pharmacol.818, 241–253. 10.1016/j.ejphar.2017.10.043 (2018). - PubMed
-
- Henriksen, P. A. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart104, 971–977. 10.1136/heartjnl-2017-312103 (2018). - PubMed
-
- Yarmohammadi, F., Rezaee, R. & Karimi, G. Natural compounds against doxorubicin-induced cardiotoxicity: a review on the involvement of Nrf2/AREsignaling pathway. Phytother. Res.35, 1163–1175. 10.1002/ptr.6882 (2021). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

