A disproportionality analysis of cardiac arrhythmia associated with bisphosphonates based on the FAERS database
- PMID: 40593869
- PMCID: PMC12218972
- DOI: 10.1038/s41598-025-00900-y
A disproportionality analysis of cardiac arrhythmia associated with bisphosphonates based on the FAERS database
Abstract
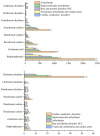
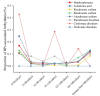
The impact of bisphosphonates on cardiac arrhythmia has always been controversial. We aimed to evaluate the risk of bisphosphonates (BPs) on arrhythmia and to characterize the main features of BP-induced arrhythmia by data mining the FDA adverse event Reporting System (FAERS). From the first quarter of 2016 to the second quarter of 2022, reports for BP-related cardiac arrhythmia adverse event (AE) from FAERS database were analyzed in this study. The reporting odds ratio (ROR) and information components (IC) were used to identify disproportionate reporting of BP-related cardiac arrhythmias. We identified a total of 2377 BP-related cardiac arrhythmias cases which appeared to influence more women (61.88%) than men (28.02%), with a median age of 70 (interquartile range [IQR] 59-78) years. Compared with the full database, BPs were detected with pharmacovigilance of cardiac arrhythmias (ROR025 = 1.43, [1.39-1.47]). All studied bisphosphonates except ibandronate sodium and clodronate disodium were correlated with the reporting frequency of cardiac arrhythmia (HLGT), with ROR ranging from 1.28 with pamidronate disodium to 11.08 with etidronate disodium. The spectrum of arrhythmias induced by BPs differed among therapeutic regimens. There were significant differences in the onset time among BP regimens. Bisphosphonates use is associated with adverse cardiac arrhythmias, with the exception of ibandronate sodium and clodronate disodium. Our data support differential effects of BPs on cardiac arrhythmias.
Keywords: Adverse event; Bisphosphonates; Cardiac arrhythmias; FAERS; Pharmacovigilance.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: Institutional review board approval was waived for this study because FAERS is a public anonymized database. Reviewer disclosures: Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Figures

Similar articles
-
Bisphosphonates for breast cancer.Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003474. doi: 10.1002/14651858.CD003474.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2012 Feb 15;(2):CD003474. doi: 10.1002/14651858.CD003474.pub3. PMID: 16034900 Updated.
-
Association Between Melatonin Receptor Agonists and Cardiac Arrhythmia; Disproportionality Analysis Studies Using Pharmacovigilance Databases.Cardiovasc Toxicol. 2025 Aug;25(8):1191-1201. doi: 10.1007/s12012-025-10029-z. Epub 2025 Jun 24. Cardiovasc Toxicol. 2025. PMID: 40550964
-
Gender and age disparities in cardiac immune-related adverse events associated with immune checkpoint inhibitors: a pharmacovigilance analysis of the FAERS database.BMJ Open. 2025 Jun 12;15(6):e090087. doi: 10.1136/bmjopen-2024-090087. BMJ Open. 2025. PMID: 40506075 Free PMC article.
-
Adverse events associated with gepants: a pharmacovigilance analysis based on the FDA adverse event reporting system.J Headache Pain. 2025 Jun 23;26(1):147. doi: 10.1186/s10194-025-02091-3. J Headache Pain. 2025. PMID: 40551098 Free PMC article.
-
Bisphosphonates for breast cancer.Cochrane Database Syst Rev. 2002;(1):CD003474. doi: 10.1002/14651858.CD003474. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003474. doi: 10.1002/14651858.CD003474.pub2. PMID: 11869664 Updated.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

