Small non-coding RNAs have predicted roles in reproductive biology and transposable element regulation in the parasitic worm Strongyloides venezuelensis
- PMID: 40593876
- PMCID: PMC12215018
- DOI: 10.1038/s41598-025-01968-2
Small non-coding RNAs have predicted roles in reproductive biology and transposable element regulation in the parasitic worm Strongyloides venezuelensis
Abstract
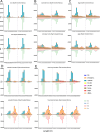
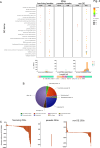
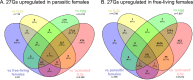
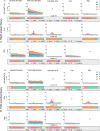
The gastrointestinal parasitic nematode Strongyloides spp. has a unique life cycle that alternates between a parasitic generation that reproduces through mitotic parthenogenesis and a dioecious free-living sexually reproducing generation. Adult females from these two generations are genetically identical, making them an informative model to identify molecular differences between parasitic and free-living lifestyles and understand different reproductive strategies. We investigated the expression of small RNAs (sRNAs) that are either enriched for a 5' monophosphate modification (5'pN) or are 5' modification-independent, across five life cycle stages of the rodent parasite Strongyloides venezuelensis. We identified miRNAs and small-interfering RNAs expressed by S. venezuelensis that are predicted to target and regulate the expression of protein-coding genes and transposable elements (TEs). Three previously unreported classes of sRNA were identified: (1) 25Gs with a putative role in reproduction in adult females, (2) tRNA-derived 24-28 nt sRNAs (tsRNAs) which are predicted to target TEs in free-living females, and (3) 5'pN-enriched 26-29Cs with 5' CGAATCC and 3' TTT motifs expressed in parasitic females. We also confirmed that S. venezuelensis expresses the 27G class of sRNAs involved in TE regulation, which was previously identified in the rodent parasite Strongyloides ratti. Taken together, these results provide new insights into the role of sRNAs in reproductive biology and parasitism.
Keywords: Strongyloides venezuelensis; Helminth; Nematode; Parasite; Small RNA; microRNA.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




References
-
- World Health Organization. 2030 Targets for Soil-Transmitted Helminthiases Control Programmes (World Health Organization, 2020).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

