Integrative machine learning and molecular simulation approaches identify GSK3β inhibitors for neurodegenerative disease therapy
- PMID: 40593894
- PMCID: PMC12217229
- DOI: 10.1038/s41598-025-04129-7
Integrative machine learning and molecular simulation approaches identify GSK3β inhibitors for neurodegenerative disease therapy
Abstract
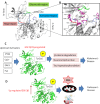
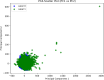
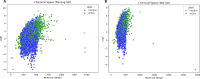
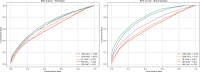
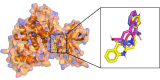
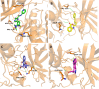
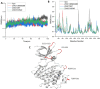
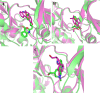
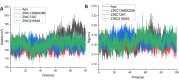
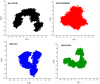
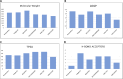
Neurodegenerative diseases (NDDs), including Alzheimer's disease (AD) and Parkinson's disease (PD), are a growing global health concern, especially among the elderly, posing significant challenges to well-being and survival. GSK3β, a serine/threonine kinase, is a key molecular player in the pathogenesis of NDDs. Dysregulated activity of GSK3β has been linked to neurodegenerative complications. Targeting GSK3β with active-site-specific inhibitors presents a promising therapeutic strategy for mitigating its pathological effects and potentially intercepting NDD progression. This study aimed to identify potential GSK3β inhibitors through an integrated in silico approach combining machine learning (ML)-based virtual screening, molecular docking, molecular dynamics (MD) simulations, and MM/GBSA binding free energy calculations. ML models were trained using known GSK3β inhibitors from BindingDB. Among all models, the Random Forest (RF) algorithm had the best prediction accuracy, with a value of 0.6832 on the test set and 0.7432 on the training set, and was employed to screen the target library of 11,032 phytochemicals. The ML-based screening identified 2,898 compounds with potential inhibitory action against GSK3β. Further screening based on Lipinski's Rule of Five gave 221 drug-like candidates. These compounds were further evaluated for GSK3β interaction via molecular docking. The analyses found ZINC136900288, ZINC7267, and ZINC519549 bind strongly and interact well with key residues in GSK3β active site with their binding scores being - 9.9, -8.8, and - 8.7 kcal/mol, respectively. MD simulations were conducted for both ligand-bound and apo GSK3β to assess structural stability. The simulation results showed that the ligand bound complexes were structurally stable with less fluctuations and higher conformational stability. In addition, (MM/GBSA) binding free energy calculations were carried out to quantify the affinity of the candidate compounds, and the candidate compound ZINC136900288 has the strongest binding affinity (-24.86 kcal/mol) of the three. Notably, these identified compounds feature novel chemical scaffolds that are structurally distinct from previously reported GSK3β inhibitors, emphasizing the originality and therapeutic potential of this study. These results show that ZINC136900288 may serve as suitable GSK3β inhibitors. Nevertheless, the efficacy and safety of these compounds need to be further validated experimentally and further studied in vivo for possible therapeutic application in NDDs.
Keywords: Alzheimer’s’ disease; Glycogen synthase kinase-3 beta; Inhibitors; Machine learning; Molecular docking; Molecular dynamics simulation; Parkinson’s disease.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Molecular docking studies and molecular dynamic simulation analysis: To identify novel ATP-competitive inhibition of Glycogen synthase kinase-3β for Alzheimer's disease.F1000Res. 2025 May 27;13:773. doi: 10.12688/f1000research.145391.3. eCollection 2024. F1000Res. 2025. PMID: 40443428 Free PMC article.
-
Identification of Novel Scaffolds Against GSK-3β for Targeting Alzheimer's Disease Through Molecular Modeling Techniques.Cell Mol Neurobiol. 2025 Aug 4;45(1):77. doi: 10.1007/s10571-025-01568-8. Cell Mol Neurobiol. 2025. PMID: 40760108 Free PMC article.
-
Evaluating the inhibitory efficacy of Oxalis phytocompounds on monoamine oxidase B: An integrated approach targeting age related neurodegenerative diseases through molecular docking and dynamics simulations.PLoS One. 2025 Jul 30;20(7):e0329168. doi: 10.1371/journal.pone.0329168. eCollection 2025. PLoS One. 2025. PMID: 40737296 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Selegiline for Alzheimer's disease.Cochrane Database Syst Rev. 2003;(1):CD000442. doi: 10.1002/14651858.CD000442. Cochrane Database Syst Rev. 2003. PMID: 12535396
References
-
- Ali, A., Hoeflich, K. P. & Woodgett, J. R. Glycogen synthase kinase-3: properties, functions, and regulation. Chem. Rev.101 (8), 2527–2540 (2001). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

