Bioinspired Conyza bonariensis-mediated ZnO/rGO NCs for effective degradation of toxic compounds under visible-light irradiation
- PMID: 40593917
- PMCID: PMC12219070
- DOI: 10.1038/s41598-025-03229-8
Bioinspired Conyza bonariensis-mediated ZnO/rGO NCs for effective degradation of toxic compounds under visible-light irradiation
Abstract
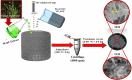
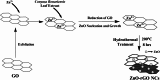
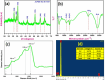
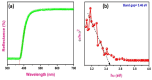
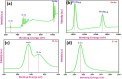
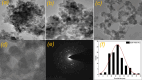
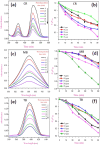
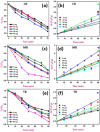
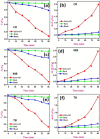
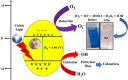
This study reports a green, cost-effective synthesis of ZnO/rGO nanocomposites (NCs) using Conyza bonariensis leaf extract as a novel bio-reducing agent. The nanocomposites were prepared via a simple hydrothermal method. Extensive characterization techniques including XRD, FT-IR, EDS, UV-DRS, XPS, BET, SEM, TEM, and AFM were employed to evaluate the crystallite size, phase structure, chemical composition, surface morphology, porosity, and particle size of the synthesized material. XRD analysis confirmed the formation of a hexagonal wurtzite ZnO phase with an average crystallite size of approximately 17.22 nm, calculated using the Debye-Scherrer equation. SEM revealed a distinctive "tuberose flower"-like morphology of ZnO particles distributed on the reduced graphene oxide (rGO) sheets, with flower diameters ranging from 1 to 2 μm and petal widths of 40-70 nm. Further, TEM supported the uniform distribution of ZnO tubular petals on graphene nanosheets. BET analysis demonstrated the mesoporous nature of NCs. Remarkably, the bioinspired ZnO/rGO NCs exhibited excellent photocatalytic activity under visible-light irradiation, effectively degrading industrial dyes such as Congo red (CR), Methylene blue (MB), and Thymol blue (TB). The enhanced photocatalytic performance is attributed to the nanocomposites' unique scaffold-like architecture, increased light absorption, and efficient charge separation.
Keywords: Bioinspired ZnO/rGO NCs; Environmental mitigation; Mesoporous material; Nature-inspired synthesis; Photocatalytic performance; Toxic dyes.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Visible-light-driven removal of mixed dye pollutants by a novel ZnO/CNT/GO ternary nanocomposite: Synergistic degradation of Congo red and methylene blue.Environ Res. 2025 Jun 14;283:122156. doi: 10.1016/j.envres.2025.122156. Online ahead of print. Environ Res. 2025. PMID: 40523584
-
Eco-friendly synthesis of ZnO nanoparticles using Siirt pistachio thin shell extract: promising applications in photocatalysis and antibacterial solutions.Int J Phytoremediation. 2025 Jul 9:1-13. doi: 10.1080/15226514.2025.2530018. Online ahead of print. Int J Phytoremediation. 2025. PMID: 40635233
-
Photocatalytic degradation activity of goji berry extract synthesized silver-loaded mesoporous zinc oxide (Ag@ZnO) nanocomposites under simulated solar light irradiation.Sci Rep. 2022 Jun 15;12(1):10017. doi: 10.1038/s41598-022-14117-w. Sci Rep. 2022. PMID: 35705651 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Synthesis, Characterization, and Application of Magnetic Zeolite Nanocomposites: A Review of Current Research and Future Applications.Nanomaterials (Basel). 2025 Jun 13;15(12):921. doi: 10.3390/nano15120921. Nanomaterials (Basel). 2025. PMID: 40559283 Free PMC article. Review.
References
-
- Khan, S. & Malik, A. Environmental and health effects of textile industry wastewater. Environmental deterioration and human health: Natural and anthropogenic determinants, 55–71. (2014). 10.1007/978-94-007-7890-0_4
-
- Kumar, R., Kaushal, S., Verma, N., Kumar, P., Thakur, N., Kumar, A., et al. Nano bioaugmentation for textile dye remediation: a sustainable approach for health and environment management. J. Mol. Liq. 126254. https://doi.org/10.1016/j.molliq.2024.126254. (2024).
-
- Zhou, H., Wang, H., Yue, C., He, L., Li, H., Zhang, H., et al. Photocatalytic degradation by TiO2-conjugated/coordination polymer heterojunction: preparation, mechanisms, and prospects. Appl. Catal. B: Environ. Energy344, 123605. https://doi.org/10.1016/j.apcatb.2023.123605. (2024).
-
- Zhang, L., Kuang, P. & Yu, J. Introductory chapter: Fundamentals of photocatalysis and electrocatalysis. In Graphene Oxide-Metal Oxide and Other Graphene Oxide-Based Composites in Photocatalysis and Electrocatalysis (pp. 1–30). Elsevier. (2022). 10.1016/B978-0-12-824526-2.00001-5
-
- Vinayagam, R., Hebbar, A., Kumar, P. S., Rangasamy, G., Varadavenkatesan, T., Murugesan,G., et al. Green synthesized cobalt oxide nanoparticles with photocatalytic activity towards dye removal. Environ. Res.216, 114766. 10.1016/j.envres.2022.114766 (2023). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

