The novel food evaluation process delays access to food innovation in the European Union
- PMID: 40593922
- PMCID: PMC12219162
- DOI: 10.1038/s41538-025-00492-x
The novel food evaluation process delays access to food innovation in the European Union
Abstract
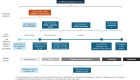
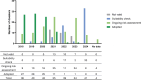
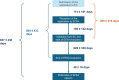
This article analyzes the timelines of 292 novel food (NF) applications submitted to EFSA between 2018 and 2024 under Regulation (EU) 2015/2283. The average duration from submission to publication was 2.56 ± 1.19 years, with significant variability, and delays due to suitability checks and additional data requests. Improved guidelines and pre-submission support could streamline the process, fostering innovation and timely market access for NFs.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: All authors are employed of FoodChain ID, a consulting company managing new ingredient authorization in Europe and in the U.S. on behalf of its clients.
Figures
Similar articles
-
Assessment of whether use of a professional medical writer is associated with time to publication of papers reporting phase III oncology clinical trials in two high-impact general medicine journals.Curr Med Res Opin. 2025 May;41(5):887-893. doi: 10.1080/03007995.2025.2505697. Epub 2025 May 23. Curr Med Res Opin. 2025. PMID: 40372172
-
Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children.Cochrane Database Syst Rev. 2012 Jan 18;1:CD008965. doi: 10.1002/14651858.CD008965.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Apr 10;(4):CD008965. doi: 10.1002/14651858.CD008965.pub4. PMID: 22258996 Updated.
-
Direct-acting antivirals for chronic hepatitis C.Cochrane Database Syst Rev. 2017 Sep 18;9(9):CD012143. doi: 10.1002/14651858.CD012143.pub3. Cochrane Database Syst Rev. 2017. PMID: 28922704 Free PMC article.
-
Ultrasound and shockwave therapy for acute fractures in adults.Cochrane Database Syst Rev. 2012 Feb 15;(2):CD008579. doi: 10.1002/14651858.CD008579.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Jun 23;(6):CD008579. doi: 10.1002/14651858.CD008579.pub3. PMID: 22336847 Updated.
-
Early additional food and fluids for healthy breastfed full-term infants.Cochrane Database Syst Rev. 2016 Aug 30;2016(8):CD006462. doi: 10.1002/14651858.CD006462.pub4. Cochrane Database Syst Rev. 2016. PMID: 27574798 Free PMC article.
References
-
- European Commission. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001 (Text with EEA relevance)Text with EEA relevance [Internet]. Mar 27, 2021. Available from: http://data.europa.eu/eli/reg/2015/2283/2021-03-27/eng.
-
- Ververis, E. et al. Novel foods in the European Union: Scientific requirements and challenges of the risk assessment process by the European Food Safety Authority. Food Res Int Ott. Ont.137, 109515 (2020). - PubMed
-
- Le Bloch, J., Rouault, M., Iriantsoa, V. & Michelet, O. Polyphenols in human nutrition: European regulations and potential classification as a novel food or food additive. J. Agric Food Chem.72, 26936–26942 (2024). - PubMed
Publication types
LinkOut - more resources
Full Text Sources

