The effects of candidate probiotic strains on the gut environment in dextran sulfate sodium-induced colitis mouse
- PMID: 40593990
- PMCID: PMC12215883
- DOI: 10.1038/s41598-025-03860-5
The effects of candidate probiotic strains on the gut environment in dextran sulfate sodium-induced colitis mouse
Abstract
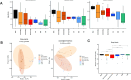
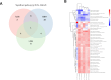
Inflammatory bowel disease (IBD) is a chronic condition characterized by intestinal inflammation and dysbiosis, with limited treatment options and significant challenges in long-term management. This study investigated the therapeutic potential of novel strains belonging to Bifidobacterium longum and Limosilactobacillus species, in a dextran sodium sulfate (DSS)-induced mouse model of colitis. In this study, our primary objective was to determine whether ingestion of these strains alleviates colitis symptoms and, if so, to elucidate how they restored gut microbial balance and modulated microbial metabolic functions. In most probiotic-treated groups, colitis disease activity index scores were significantly improved and colon length was preserved, with strains CBA7106 and BBH exhibiting efficacy comparable to that of the Lactobacillus rhamnosus GG (used as a positive control). Moreover, histological analyses confirmed reduced inflammation and enhanced mucosal integrity. Microbial diversity assessments demonstrated a marked restoration of gut microbial composition, highlighted by increased abundances of beneficial taxa such as Lactobacillus and Akkermansia. Metabolomic profiling identified key anti-inflammatory metabolites, including 6-hydroxycaproic acid, indole-3-lactic acid, and choline, which were significantly elevated in the probiotic-treated groups. Notably, functional analyses using PICRUSt2 revealed a sustained decrease in the siderophore biosynthesis pathway (ko01053), suggesting that these candidate probiotic strains may inhibit siderophore production-a pivotal mechanism by which pathogenic bacteria aggravate intestinal inflammation. Taken together, these findings indicate that the candidate probiotic strains CBA7106 and BBH effectively mitigate DSS-induced colitis by modulating the gut microbiota, promoting the production of anti-inflammatory metabolites, and suppressing siderophore biosynthesis. This study provides valuable insights into the development of targeted probiotic therapies for IBD, underscoring their potential as a complementary approach to restoring intestinal health and reducing inflammation. Further clinical studies are warranted to validate these observations in human populations.
Keywords: Candidate probiotic strains; Colitis; Dextran sulfate sodium; Gut microbiota; Inflammatory bowel disease.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper Ethical approval: All animal experiments were approved by the Institutional Animal Care and Use Committee of Gyeongsang National University (GNU-220522-M0053).
Figures

Similar articles
-
Lactobacillus rhamnosus KBL2290 Ameliorates Gut Inflammation in a Mouse Model of Dextran Sulfate Sodium-Induced Colitis.J Microbiol. 2023 Jul;61(7):673-682. doi: 10.1007/s12275-023-00061-5. Epub 2023 Jun 14. J Microbiol. 2023. PMID: 37314676
-
Gut microbial Nordihydroguaiaretic acid suppresses macrophage pyroptosis to regulate epithelial homeostasis and inflammation.Gut Microbes. 2025 Dec;17(1):2518338. doi: 10.1080/19490976.2025.2518338. Epub 2025 Jul 1. Gut Microbes. 2025. PMID: 40596758 Free PMC article.
-
Multiomics Analysis of Bacteroides cellulosilyticus Anticolitis via Gut Microbiota Metabolite-Mediated PI3K-Akt Signaling Pathway.J Agric Food Chem. 2025 Jul 2;73(26):16333-16347. doi: 10.1021/acs.jafc.5c00637. Epub 2025 Jun 20. J Agric Food Chem. 2025. PMID: 40539526
-
The Role of Genetically Engineered Probiotics for Treatment of Inflammatory Bowel Disease: A Systematic Review.Nutrients. 2023 Mar 24;15(7):1566. doi: 10.3390/nu15071566. Nutrients. 2023. PMID: 37049407 Free PMC article.
-
Probiotics for management of functional abdominal pain disorders in children.Cochrane Database Syst Rev. 2023 Feb 17;2(2):CD012849. doi: 10.1002/14651858.CD012849.pub2. Cochrane Database Syst Rev. 2023. PMID: 36799531 Free PMC article.
References
-
- Larsen, S., Bendtzen, K. & Nielsen, O. H. Extraintestinal manifestations of inflammatory bowel disease: Epidemiology, diagnosis, and management. Ann. Med.42, 97–114 (2010). - PubMed
-
- Neurath, M. F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol.14, 269–278 (2017). - PubMed
-
- Ananthakrishnan, A. N. et al. Environmental triggers in IBD: A review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol.15, 39–49 (2018). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

