Preparation and property evaluation of oral colon targeted protein delivery system with sodium alginate and chitosan
- PMID: 40593991
- PMCID: PMC12218344
- DOI: 10.1038/s41598-025-04983-5
Preparation and property evaluation of oral colon targeted protein delivery system with sodium alginate and chitosan
Abstract
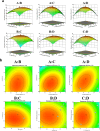
Oral administration of protein-based biologics faces challenges such as low bioavailability and a short half-life in the gastrointestinal tract. This study focuses on developing a novel encapsulation technique using sodium alginate and chitosan to create nanospheres that efficiently deliver bovine serum albumin, a model protein. Through a combination of single-factor and response surface experiments, optimal preparation conditions were identified, yielding stable nanospheres with high encapsulation rates and small particle sizes. The release of the protein was pH-dependent, with more substantial release under alkaline conditions, resembling the environment of the colon. In vitro safety testing confirmed that the nanospheres had low toxicity and did not induce significant hemolysis or cell death. This approach demonstrates significant potential for enhancing the oral bioavailability of protein-based drugs, overcoming the typical challenges faced by oral drug delivery systems. The developed formulation offers a simple yet effective method for targeted colonic delivery, representing a promising strategy for improving the clinical efficiency of protein biologics.
Keywords: Biosafety; Protein-based biologics; Response surface experiments; SA-BSA-CS; Single-factor experiment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Effect of processing conditions on the physical-chemical and mechanical properties of chitosan-alginate polyelectrolyte complex films for potential wound dressing application.J Biomater Appl. 2025 Aug;40(2):236-251. doi: 10.1177/08853282251334472. Epub 2025 Apr 14. J Biomater Appl. 2025. PMID: 40226979
-
Structural studies on the interaction of CTAB with alginate: Possibility of surfactant therapy with chemo sensitization effect.J Pharm Sci. 2025 Apr;114(4):103701. doi: 10.1016/j.xphs.2025.103701. Epub 2025 Feb 13. J Pharm Sci. 2025. PMID: 39954808
-
Superior growth performance in carp fry achieved with chitosan-alginate encapsulated A-ghrelin versus free peptide: Evidence from physiological, molecular, and morphological analyses.PLoS One. 2025 Jun 30;20(6):e0327235. doi: 10.1371/journal.pone.0327235. eCollection 2025. PLoS One. 2025. PMID: 40587525 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- STERGIOPOULOS, S. et al. Adverse drug reaction case safety practices in large biopharmaceutical organizations from 2007 to 2017: an industry Survey[J]. Pharm. Med.33 (6), 499–510 (2019). - PubMed
-
- JIN, C. et al. Efficacy and safety of PD-1/PD-L1 and CTLA-4 immune checkpoint inhibitors in colorectal cancer: a meta-analysis[J]. J. Comp. Eff. Res.11 (3), 203–212 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

