Biofilm detachment significantly affects biological stability of drinking water during intermittent water supply in a pilot scale water distribution system
- PMID: 40594086
- PMCID: PMC12215843
- DOI: 10.1038/s41598-025-03535-1
Biofilm detachment significantly affects biological stability of drinking water during intermittent water supply in a pilot scale water distribution system
Abstract
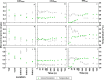
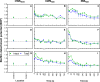
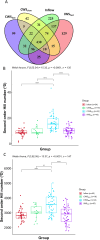
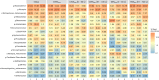
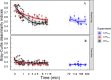
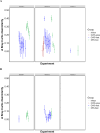
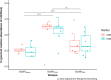
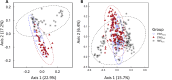
Intermittent service provision (IWS) in piped drinking water distribution systems is practiced in countries with limited water resources; it leads to stagnant periods during which water drains completely from de-pressurized pipes, increasing the likelihood of biofilm detachment upon reconnection when water is supplied to the consumer and thus affecting water quality. Our study examines the impact of uninterrupted or continuous water supply (CWS) and IWS on microbial communities and biofilm detachment, using data from three 30-day experiments conducted in an above-ground drinking water testbed with 90-m long PVC pipes containing residual monochloramine. Flow cytometry (FCM) revealed a significant increase in total and intact cell concentrations when water was supplied intermittently compared to CWS, and the microbial alpha-diversity was significantly higher in CWS sections by both 16S rRNA gene metabarcoding and phenotypic fingerprinting of flow cytometry data. Nitrate levels in the water were significantly higher during initial intermittent flow due to the activity of nitrifying bacteria in biofilms exposed to stagnant water in pipes. Overall, biofilm detachment significantly affects the biological stability of drinking water delivered through IWS compared to CWS. We developed a novel biofilm detachment potential index derived from FCM data to estimate the minimum amount of water needed to be discarded before microbial cell counts and community composition return to baseline levels.
Keywords: Drinking water Microbiome; Drinking water biofilms; Intermittent water supply; Monochloramine; Nitrification; Phenotypic fingerprinting.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda, 4 edn (World Health Organization, 2022). - PubMed
-
- Bivins, A. W. et al. Estimating Infection Risks and the Global Burden of Diarrheal Disease Articulable to Intermittent Water Supply Using QMRA (2017). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

