Strictosamide and mitraphylline inhibit cancer cell motility by suppressing epithelial-mesenchymal transition via integrin α4-mediated signaling
- PMID: 40594088
- PMCID: PMC12217235
- DOI: 10.1038/s41598-025-04064-7
Strictosamide and mitraphylline inhibit cancer cell motility by suppressing epithelial-mesenchymal transition via integrin α4-mediated signaling
Abstract
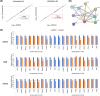
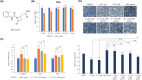
Epithelial-mesenchymal transition (EMT) is a critical process in cancer cell motility and metastasis. Monoterpene indole alkaloids (MIAs) have been widely investigated for biological activities, but rarely been explored for cell motility inhibition. This study aimed to discover natural products, especially focusing on MIAs, that inhibit EMT in cancer cells, based on our screening using multiple plant extracts. We found that an extract of the aerial parts of Uncaria scandens (Sm.) Wall. (Rubiaceae) decreased cancer cell motility. Targeted isolation exhibited eight MIAs. Among them, strictosamide and mitraphylline suppressed the invasion and migration of the cells. The alteration of the mRNA expression of the EMT effectors and transcription factors suggested that the EMT signaling pathway is related to the suppression of cancer cell motility by both compounds. RT-PCR gene array suggested inhibition of integrin α4 signaling as a potential mechanism of the EMT inhibition, which was supported by the quantitative analysis of the mRNA expressions of the related genes. Together, present study is the first report highlighting the cell motility-suppressive effects of strictosamide and mitraphylline. Our results suggested the potential applicability of strictosamide and mitraphylline for the prevention of metastasis.
Keywords: Bioactivity-guided fractionation; Integrin Α4β1 agonist; Metastasis suppressor; Spirocylic oxindole alkaloid; Vallesiachotaman-type alkaloid.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
An update review on monoterpene indole alkaloids and biological activities of Tabernaemontana species occurring in Brazil.J Ethnopharmacol. 2024 Jun 28;328:117921. doi: 10.1016/j.jep.2024.117921. Epub 2024 Feb 16. J Ethnopharmacol. 2024. PMID: 38369065 Review.
-
Inhibition of Transforming Growth Factor-β (TGF-β) Signaling by Scutellaria baicalensis and Fritillaria cirrhosa Extracts in Endometrial Cancer.J Cell Biochem. 2015 Aug;116(8):1797-805. doi: 10.1002/jcb.25138. J Cell Biochem. 2015. PMID: 25683036
-
TGF-β1 exposure induces epithelial to mesenchymal transition both in CSCs and non-CSCs of the A549 cell line, leading to an increase of migration ability in the CD133+ A549 cell fraction.Cell Death Dis. 2013 May 2;4(5):e620. doi: 10.1038/cddis.2013.144. Cell Death Dis. 2013. PMID: 23640462 Free PMC article.
-
Monensin suppresses EMT-driven cancer cell motility by inducing Golgi pH-dependent exocytosis of GOLIM4.Proc Natl Acad Sci U S A. 2025 Jul 15;122(28):e2501347122. doi: 10.1073/pnas.2501347122. Epub 2025 Jul 9. Proc Natl Acad Sci U S A. 2025. PMID: 40632561
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

