Hydroxytyrosol induced ferroptosis through Nrf2 signaling pathway in colorectal cancer cells
- PMID: 40594119
- PMCID: PMC12216606
- DOI: 10.1038/s41598-025-04415-4
Hydroxytyrosol induced ferroptosis through Nrf2 signaling pathway in colorectal cancer cells
Abstract
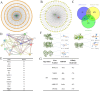
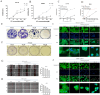
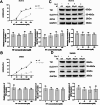
In recent years, the incidence of colorectal cancer is still on the rise. The killing of tumor cells through chemotherapy and/or radiation therapy is the mainstay of clinical anticolorectal cancer therapy, but is limited by drug and radiation resistance of tumor cells. Ferroptosis, a novel mode of programmed cell death, plays an important role in antitumor therapy. Ferroptosis inducers have been extensively studied as a strategy to target drug-resistant cancers. The aim of this study is to investigate the mechanism by which hydroxytyrosol (HT) induces ferroptosis in colorectal cancer cells via the Nrf2 signaling pathway. The goal of this study is to use network pharmacology and molecular docking approaches to screen and confirm hydroxytyrosol targets for the treatment of colorectal cancer. The response of colorectal cancer cells to hydroxytyrosol was assessed by cell viability, colony formation assay and scratch assay. Additionally, molecular techniques, including Western blotting and fluorescent probe technology, were employed. The network pharmacological screen identified 14 core targets. Among these genes, nuclear factor-erythroid 2 related factor 2 (Nrf2) was identified as the top target. Molecular docking revealed enhanced binding activity for HT with targets related to oxidative stress, including Nrf2, NAD(P)H quinone oxidoreductase 1 (NQO1), thioredoxin reductase 1 (TrxR1), prostaglandin-endoperoxide synthase 2 (PTGS2) and aldo-keto reductase 1C3 (AKR1C3). HT-induced ferroptosis elevates iron levels, lipid peroxidation (LPO) and reactive oxygen species (ROS), while decreasing glutathione (GSH) and mitochondrial membrane potential. Moreover, HT reduced the expression of solute carrier family 7 member 11 (SLC7A11) and glutathione peroxidase 4 (GPX4) proteins while increasing the expression of Tfr1 protein. Changes in the expression levels of these proteins led to an increase in soluble iron pools, which in turn promoted lipid peroxidation. Notably, the ferritin deposition inhibitor ferroprostatin-1 (Fer-1) significantly reversed this process. Additionally, the levels of protein expression of Nrf2 and NQO1 were reversed by two activators of Nrf2, bardoxolone (CDDO) and sulforaphane (SFN). In summary, we provide evidence that HT may induce ferroptosis in colorectal cancer cells. Mechanistically, HT induces ferroptosis via the Nrf2 signaling pathway.
Keywords: Colorectal cancer; Ferroptosis; Hydroxytyrosol; Nrf2 signaling pathway.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Aldo-keto Reductase 1B10 (AKR1B10) Suppresses Sensitivity of Ferroptosis in TNBC by Activating the AKT/GSK3β/Nrf2/GPX4 Axis.Front Biosci (Landmark Ed). 2025 Jun 27;30(6):36615. doi: 10.31083/FBL36615. Front Biosci (Landmark Ed). 2025. PMID: 40613296
-
Cadmium Inhibits Proliferation of Human Bronchial Epithelial BEAS-2B Cells Through Inducing Ferroptosis via Targeted Regulation of the Nrf2/SLC7A11/GPX4 Pathway.Int J Mol Sci. 2025 Jul 25;26(15):7204. doi: 10.3390/ijms26157204. Int J Mol Sci. 2025. PMID: 40806353 Free PMC article.
-
Paclitaxel Attenuates Atherosclerosis by Suppressing Macrophage Ferroptosis and Improving Lipid Metabolism via the Sirt1/Nrf2/GPX4 Pathway.FASEB J. 2025 Aug 15;39(15):e70917. doi: 10.1096/fj.202501047RR. FASEB J. 2025. PMID: 40779351
-
Ferroptosis and Nrf2 Signaling in Head and Neck Cancer: Resistance Mechanisms and Therapeutic Prospects.Antioxidants (Basel). 2025 Aug 13;14(8):993. doi: 10.3390/antiox14080993. Antioxidants (Basel). 2025. PMID: 40867889 Free PMC article. Review.
-
Targeting ferroptosis using Chinese herbal compounds to treat respiratory diseases.Phytomedicine. 2024 Jul 25;130:155738. doi: 10.1016/j.phymed.2024.155738. Epub 2024 Jun 1. Phytomedicine. 2024. PMID: 38824825
References
-
- Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.74 (3), 229–263 (2024). - PubMed
-
- Kong, M. Y. et al. Chinese herbal medicines for prevention and treatment of colorectal cancer: from molecular mechanisms to potential clinical applications. J. Integr. Med.18 (5), 369–384 (2020). - PubMed
MeSH terms
Substances
Grants and funding
- 23JRRA1254/the Gansu Provincial Science and Technology Programme
- 2022-4-56/the Lanzhou Municipal Science and Technology Programme
- 2022-3-39/the Lanzhou Municipal Science and Technology Programme
- 31960141/The National Natural Science Foundation of China
- 2018-1-122/the Lanzhou Municipal Science and Technology Development Programme
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

