Synthesis and characterization of biobased capsules formed from interpenetrating networks of alginate and poly(ethylene glycol) for the encapsulation of blue dye
- PMID: 40594413
- PMCID: PMC12215545
- DOI: 10.1038/s41598-025-05352-y
Synthesis and characterization of biobased capsules formed from interpenetrating networks of alginate and poly(ethylene glycol) for the encapsulation of blue dye
Abstract
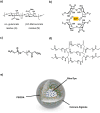
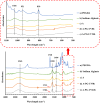
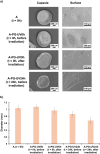
Encapsulation technologies have been utilized in laundry detergents mainly as formaldehyde-based capsules which are commonly used to encapsulate active compounds like fragrances, bluing agents and fluorescent whitening agents. Nevertheless, formaldehyde derived materials are toxic, carcinogenic, and non-biodegradable leading to an increase in the microplastic pollution in oceans and consequently harming the marine life. Therefore, the researchers are currently tending towards the replacement of these components by biobased ones. In this work, we present the synthesis of capsules with more than one shell using biodegradable polymers to replace these materials. Moreover, the blue dye used in laundry detergent industry was successfully encapsulated in biodegradable capsules formed by an interpenetrating network of alginate and poly(ethylene glycol) diacrylate (PEGDA) prepared using different conditions. Besides, the capsules were characterized to study their chemical, morphological, thermal, and mechanical properties, to evaluate their water solubility, and to determine how the composition and the preparation methods can affect their properties. The novelty of this system lies in evaluating how modifying a previously reported system using poly(ethylene glycol) dimethacrylate (PEGDMA) and alginate as shells -achieved by replacing the PEGDMA diacrylic monomer with PEGDA- affects the morphology and properties of the resulting capsules. It has been shown that the capsules with PEGDA exhibited improved thermal and mechanical properties compared to the previously described system, which could make them more suitable for their intended applications.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Aguiar, A., Loureiro, M. V., Pinho, I. & Marques, A. C. Efficient encapsulation of isocyanates in PCL/PLA biodegradable microcapsules for adhesives. J. Mater. Sci.58, 2249–2267 (2023).
-
- Machado, T. O. et al. Biopolymer-based nanocarriers for sustained release of agrochemicals: A review on materials and social science perspectives for a sustainable future of agri- and horticulture. Adv. Colloid Interface Sci.303, 102645 (2022). - PubMed
-
- Tomadoni, B., Fabra, M. J. & López-Rubio, A. Electrohydrodynamic processing of phycocolloids for food-related applications: recent advances and future prospects. Trends Food Sci. Technol.125, 114–125 (2022).
Grants and funding
- PID2020-116322RB-C32/Ministerio de Ciencia e Innovación
- PID2020-116322RB-C32/Ministerio de Ciencia e Innovación
- PID2020-116322RB-C32/Ministerio de Ciencia e Innovación
- PID2020-116322RB-C32/Ministerio de Ciencia e Innovación
- 2021PMF-PIPF-24/Universitat Rovira i Virgili, Marti i Franquès pre-doctoral contract
- 2021PMF-PIPF-24/Universitat Rovira i Virgili, Marti i Franquès pre-doctoral contract
- 2021PMF-PIPF-24/Universitat Rovira i Virgili, Marti i Franquès pre-doctoral contract
- 2021PMF-PIPF-24/Universitat Rovira i Virgili, Marti i Franquès pre-doctoral contract
- Marie Skłodowska-Curie grant agreement No. 713679/European Union's Horizon 2020 research and innovation programme
- Marie Skłodowska-Curie grant agreement No. 713679/European Union's Horizon 2020 research and innovation programme
- Marie Skłodowska-Curie grant agreement No. 713679/European Union's Horizon 2020 research and innovation programme
LinkOut - more resources
Full Text Sources

