Anisotropic chemical bonding of lanthanide-OH molecules
- PMID: 40594426
- PMCID: PMC12216299
- DOI: 10.1038/s41598-025-06281-6
Anisotropic chemical bonding of lanthanide-OH molecules
Abstract
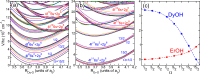
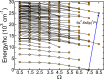
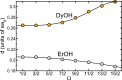
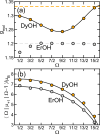
We present a theoretical study of the low lying adiabatic relativistic electronic states of lanthanide monohydroxide (Ln-OH) molecules near their linear equilibrium geometries. In particular, we focus on heavy, magnetic DyOH and ErOH relevant to fundamental symmetry tests. We use a restricted-active-space self-consistent field method combined with spin-orbit coupling as well as a relativistic coupled-cluster method to determine ground and excited electronic states. In addition, electric and magnetic dipole moments are computed with the self-consistent field method. Analysis of the results from both methods shows that the dominant molecular configuration of the ground state is one where an electron from the partially filled and anisotropic 4f orbital of the lanthanide atom moves to the hydroxyl group, leaving the closed outer-most [Formula: see text] lone electron pair of the lanthanide atom intact in sharp contrast to the bonding in alkaline-earth monohydroxides and YbOH, where an electron from the outer-most s shell moves to the hydroxyl group. For linear molecules the projection of the total electron angular momentum on the symmetry axis is a conserved quantity with quantum number Ω and we study the polynomial Ω dependence of the energies of the ground states as well as their electric and magnetic moments. We find that for both molecules Ω lies between [Formula: see text] and [Formula: see text], where the degenerate states with the lowest energy have [Formula: see text] and 1/2 for DyOH and ErOH, respectively. The zero field splittings among these Ω states is approximately [Formula: see text] [Formula: see text], where h is the Planck constant and c is the speed of light in vacuum. We find that the permanent dipole moments for both triatomics are fairly small at 0.23 atomic units and are mostly independent of Ω. The magnetic moments are closely related to that of the corresponding atomic [Formula: see text] ion in an excited electronic state. From the polynomial Ω dependences, we also realize that the total electron angular momentum is to good approximation conserved and has a quantum number of 15/2 for both triatomic molecules. We describe how this observation can be used to construct effective Hamiltonians containing spin-spin operators.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
References
-
- Lu, M., Burdick, N. Q., Youn, S. H. & Lev, B. L. Strongly dipolar Bose–Einstein condensate of Dysprosium. Phys. Rev. Lett.107, 190401. 10.1103/PhysRevLett.107.190401 (2011). - PubMed
-
- Lu, M., Burdick, N. Q. & Lev, B. L. Quantum degenerate dipolar Fermi gas. Phys. Rev. Lett.108, 215301. 10.1103/PhysRevLett.108.215301 (2012). - PubMed
-
- Frisch, A. et al. Narrow-line magneto-optical trap for erbium. Phys. Rev. A85, 051401. 10.1103/PhysRevA.85.051401 (2012).
-
- Aikawa, K. et al. Bose–Einstein condensation of erbium. Phys. Rev. Lett.108, 210401. 10.1103/PhysRevLett.108.210401 (2012). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

