Effects of piperine on intestinal permeation, pharmacodynamics and pharmacokinetics of insulin loaded chitosan coated solid lipid nanoparticles in rats
- PMID: 40594447
- PMCID: PMC12218416
- DOI: 10.1038/s41598-025-05137-3
Effects of piperine on intestinal permeation, pharmacodynamics and pharmacokinetics of insulin loaded chitosan coated solid lipid nanoparticles in rats
Abstract
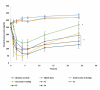
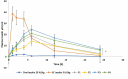
The oral administration of insulin offers significant clinical benefits due to its simplicity. This study evaluated piperine's efficacy in improving insulin's intestinal absorption in Wistar rats. Confocal microscopy demonstrated that prolonged retention of insulin-loaded chitosan-coated solid lipid nanoparticles (Ch-In-SLNs) on intestinal mucosa was due to chitosan's mucoadhesive properties. These studies also revealed Ch-In-SLNs permeation in rat intestinal segments. Piperine enhanced the permeation of insulin-loaded nanoparticles, with more particles transported into deeper intestinal layers. The study assessed Ch-In-SLNs with piperine administered orally to diabetic rats. The formulated SLN's effectiveness was evaluated in vivo in overnight-fasted Wistar rats. Male Wistar rats (n = 5) were divided into 8 groups, with the first as diabetic control, and others receiving various treatments, including blank SLN, oral insulin solution (25 IU/kg), subcutaneous (SC) insulin (5 IU/kg), and insulin nanoformulations (25 IU/kg and 50 IU/kg with and without piperine, respectively). Ch-In-SLN with piperine showed a statistically significant reduction (p < 0.05) in blood glucose levels in streptozotocin-induced (STZ) diabetic rats compared to SLNs without piperine and SC insulin. The Ch-In-SLN with piperine formulation demonstrated a noteworthy correlation between pharmacokinetics (PK) and pharmacodynamics (PD). This approach could replace traditional insulin injections and mitigate associated side effects, simplifying insulin therapy.
Keywords: Diabetes; In vivo; Insulin; Permeation enhancer; Piperine; Solid lipid nanoparticles; Streptozotocin; Wistar rats.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: The study was approved by IAEC (NGSMIPS/IAEC/JAN-2023/333) and conducted according to the CPCSEA guidelines. All of the experiments conducted on animals in this study received prior approval from the Institutional Animal Ethics Committee (IAEC), Institutional Animal Ethics Committee of the NGSM Institute of Pharmaceutical Sciences, Mangalore, India, and they followed the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) protocols. Informed consent: There are no human subjects in this article and informed consent is not applicable. Consent for publication: All the authors have read and agreed to the final copy of the finding as contained in the manuscript.
Figures





References
-
- Rahman, S. K. & Kawatra, P. Insulin delivery: what is new in the queue? Int. J. Basic. Clin. Pharmacol.6, 229 (2017). - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

