A 22 month prospective assessment of neutralizing and IgG antibody levels against SARS-CoV-2 variants following homologous and heterologous BNT162b2 boosting
- PMID: 40594462
- PMCID: PMC12219100
- DOI: 10.1038/s41598-025-05377-3
A 22 month prospective assessment of neutralizing and IgG antibody levels against SARS-CoV-2 variants following homologous and heterologous BNT162b2 boosting
Abstract
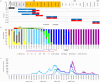
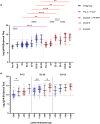
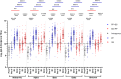
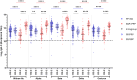
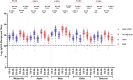
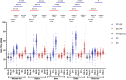
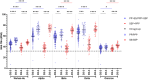
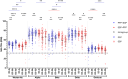
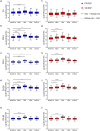
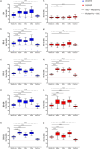
This 22-month cohort study investigated the durability and dynamics of humoral immune responses in 327 Malaysians vaccinated with either CoronaVac or BNT162b2 in early 2021, monitored up to 52 weeks following a BNT162b2 booster. Using the ImmuSAFE™ COVID + microarray, we evaluated IgG-S and NAb against Wuhan-Hu and VoCs (Alpha, Beta, Delta, Omicron), as well as IgG-N responses to Wuhan-Hu. BNT162b2 induced higher IgG-S levels and seropositivity than CoronaVac after primary immunization. While a heterologous CoronaVac-BNT162b2 temporarily increased IgG-S levels, long-term IgG-S levels and seropositivity remained comparable between the two groups across all variants. NAb levels were higher in BNT162b2 recipients than in CoronaVac recipients, with homologous BNT162b2 boosters sustaining higher NAb levels for Wuhan-Hu compared to heterologous BNT162b2-CoronaVac. However, responses to other variants varied. Expectedly, CoronaVac uniquely induced IgG-N responses, resulting in elevated IgG-N levels in heterologous BNT162b2-CoronaVac recipients compared to homologous BNT162b2 recipients. Overall, BNT162b2 boosters conferred robust and durable antibody responses regardless of the primary vaccine used. Higher IgG-N levels prior to immunization, compared to pre-pandemic levels, suggest that prior exposure to circulating SARS-CoV-2 VoCs (other than Wuhan-Hu) in Malaysia shaped immune imprinting, influencing responses before immunization with the Wuhan-Hu-based vaccine. Consequently, this study reports higher IgG-S and NAb levels against VoCs-except Omicron-compared to Wuhan-Hu after COVID-19 vaccination.
Keywords: BNT162b2; CoronaVac; Homologous and heterologous booster; IgG-S and IgG-N antibodies; Neutralizing antibodies; Variants of concern.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: NDA, T-MT are employees of Sengenics Corporation. All authors declare no financial or non-financial competing interests.
Figures

Similar articles
-
Comparison of SARS-CoV-2 IgG responses in hemodialysis patients and healthcare workers after COVID-19 vaccination.Front Immunol. 2025 Jul 15;16:1586468. doi: 10.3389/fimmu.2025.1586468. eCollection 2025. Front Immunol. 2025. PMID: 40735320 Free PMC article.
-
The impact of vaccine booster doses on specific B- and T-lymphocyte dynamics in Thai healthcare personnel following COVID-19 vaccination.Sci Rep. 2025 Jul 16;15(1):25713. doi: 10.1038/s41598-025-10400-8. Sci Rep. 2025. PMID: 40670524 Free PMC article.
-
Neutralizing and binding antibody dynamics following primary and booster COVID-19 vaccination among healthcare workers.BMC Infect Dis. 2025 Feb 14;25(1):218. doi: 10.1186/s12879-025-10621-2. BMC Infect Dis. 2025. PMID: 39953414 Free PMC article.
-
Efficacy and safety of COVID-19 vaccines.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD015477. doi: 10.1002/14651858.CD015477. Cochrane Database Syst Rev. 2022. PMID: 36473651 Free PMC article.
-
Immunogenicity and reactogenicity of heterologous immunization schedules with COVID-19 vaccines: a systematic review and network meta-analysis.Chin Med J (Engl). 2023 Jan 5;136(1):24-33. doi: 10.1097/CM9.0000000000002567. Chin Med J (Engl). 2023. PMID: 36723872 Free PMC article.
References
-
- Tin, T. P. COVID-19 cases and deaths by age (Jan 2020–Feb 3, - CodeBlue. (2023). https://codeblue.galencentre.org/2023/02/covid-19-cases-and-deaths-by-ag... (2023).
-
- Bernama DCA approves conditional registration for Vero Cell vaccine. New Straits Times Online (2021). https://www.nst.com.my/news/nation/2021/04/684816/dca-approves-condition....
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

