Combined treatment using bismuth sulfide nanoparticles loaded with NANOG decoy oligodeoxynucleotides under X-ray radiation for breast cancer cells
- PMID: 40594465
- PMCID: PMC12219885
- DOI: 10.1038/s41598-025-05074-1
Combined treatment using bismuth sulfide nanoparticles loaded with NANOG decoy oligodeoxynucleotides under X-ray radiation for breast cancer cells
Abstract
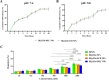
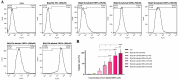
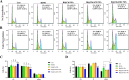
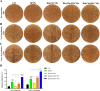
Our goal in this study was to develop bismuth sulfide nanoparticles (NPs) that were functionalized with chitosan and incorporated with decoy oligodeoxynucleotides (ODNs) specifically targeting the NANOG transcription factor (designated as Bi@Chi-DEC NPs) in triple-negative breast cancer cells. FT-IR, UV-vis, FESEM, EDX, TEM, DLS, release kinetics, and hemolysis assays were done to validate the successful synthesis of Bi@Chi-DEC NPs. The synthesized spherical particles exhibited a size distribution averaging 213.8 nm, with a zeta potential measured at -3.27 mV. The anticancer properties of the synthesized nanoparticles, along with X-ray irradiation (2Gy), were assessed through a series of cellular assays, including MTT, cellular uptake, apoptosis, cell cycle analysis, scratch, and tumorsphere formation assays on MDA-MB-231 breast cancer cells. Treatment with the synthesized nanoparticles and X-irradiation resulted in a significant reduction in cell viability, tumorsphere formation, and cellular migration, while concurrently enhancing the rate of apoptotic cells and inducing cell cycle arrest at the G2/M phase. It can be inferred that Bi@Chi-DEC NPs possess the potential to serve as a therapeutic modality for cancer treatment, particularly when utilized along with radiation therapy. Further, in vivo studies are warranted to substantiate the efficacy of this therapeutic approach.
Keywords: Bismuth nanoparticle; Breast cancer; Combinational therapy; Decoy oligodeoxynucleotides (ODNs); NANOG transcription factor; Radiotherapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: Informed consent was obtained from all individual participants included in the study. This study was approved by the Research Ethics Committees of Zanjan University of Medical Sciences, Zanjan, Iran (Ethical Code: IR.ZUMS.REC.1400.518). Moreover, all methods were performed in accordance with the relevant guidelines and regulations.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

