Comparative efficacy of clindamycin phosphate with benzoyl peroxide versus clindamycin phosphate with adapalene in acne vulgaris: a systematic review and meta-analysis
- PMID: 40594517
- PMCID: PMC12218347
- DOI: 10.1038/s41598-025-05543-7
Comparative efficacy of clindamycin phosphate with benzoyl peroxide versus clindamycin phosphate with adapalene in acne vulgaris: a systematic review and meta-analysis
Abstract
Acne vulgaris is a common skin condition that significantly impacts both physical appearance and mental well-being. Acne, being a chronic skin condition, often requires continuous treatment. This study aims to evaluate the efficacy and safety of clindamycin phosphate 1.2%/benzoyl peroxide 3% compared to clindamycin phosphate 1.2%/adapalene 0.1% combinations for treating acne vulgaris. A systematic review and meta-analysis of randomized controlled trials were carried out following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, and three databases were searched to identify RCTs comparing CLIN/BPO with CLIN/ADAP. Primary outcomes included treatment-emergent adverse events, inflammatory and non-inflammatory lesion counts, and application site side effects. Statistical analyses were conducted using RevMan 5.3. The study included a total of 800 participants across three RCTs. The meta-analysis of three RCTs demonstrated a significantly lower risk of TEAEs with CLIN/BPO (OR = 0.49, 95% CI: 0.35-0.86, p < 0.001). CLIN/BPO also resulted in fewer application site side effects (OR = 0.33, 95% CI: 0.23-0.47, p < 0.001). However, no significant differences were observed between the groups for reducing inflammatory (MD = 1.34, 𝑝 = 0.121) or non-inflammatory lesion counts (MD = 0.04, 𝑝 = 0.98). The study concluded that although CLIN/BPO was associated with fewer side effects, both treatments were equally effective in reducing acne lesions. The favorable safety profile of CLIN/BPO, particularly regarding treatment-emergent and application-site adverse events, suggests it may be the more tolerable option for patients. Future studies with larger, more diverse populations are recommended to confirm these findings and explore long-term efficacy.
Keywords: Acne vulgaris; Benzoyl peroxide; Clindamycin phosphate; Efficacy; Meta-analysis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
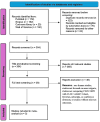


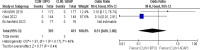
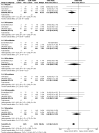
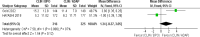
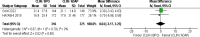
References
-
- Del Rosso, J. Q. & Schmidt, N. F. A review of the anti-inflammatory properties of clindamycin in the treatment of acne vulgaris. Cutis85 (1), 15–24 (2010). - PubMed
-
- Zaenglein, A. L. et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol.74 (5), 945–973e33 (2016). - PubMed
-
- Yentzer, B. A. et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis86 (2), 103–108 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

