Assessment of ecofriendly carbon capture using Bacillus subtilis induced calcium carbonate precipitation with focus on applications mechanisms and cost efficiency
- PMID: 40594560
- PMCID: PMC12215896
- DOI: 10.1038/s41598-025-06688-1
Assessment of ecofriendly carbon capture using Bacillus subtilis induced calcium carbonate precipitation with focus on applications mechanisms and cost efficiency
Abstract
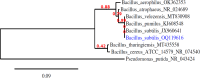
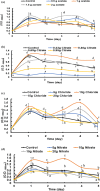
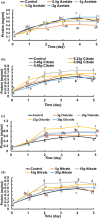
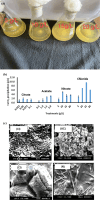
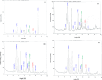
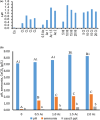
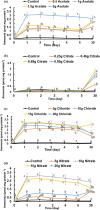
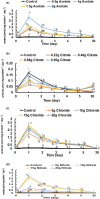
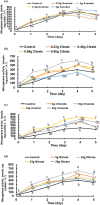
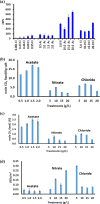
This work focuses on exploiting the naturally occurring microbial calcium carbonate precipitation catalyzed by microbial consortia within lakes and oceans biogeochemistry for carbon dioxide removal from atmosphere. In this work, Bacillus subtilis OQ119616 was used for carbon dioxide sequestration in equi-molar concentrations into Bacillus-induced calcium carbonate precipitation (BICCP). As this process requires alkaline media, urea degradation by urease and nitrogen fixation were traced. BICCP has been formed from calcium salts in the following order: chloride > nitrate > acetate > citrate. However, conversion efficiency percentage (CE%) of calcium salts to CaCO3 exhibited a different attitude of citrate > acetate > chloride > nitrate. Calcium citrate is excluded from consideration. Acetate, however, is the most efficient salt; it significantly exhibited the highest CE%, with the least cost and highest economic feasibility. The wide range in quantities, efficiency and feasibility indicates the importance of the salt anion in BICCP. In addition, BICCP exhibited applicability in healing concrete cracks, improving field capacity of sand soil and the subsequently improved seed germination of Vicia faba. BICCP was also accompanied by adsorption of heavy metals as partial purging of waste/sewage water for hygiene/reuse. Bacillus subtilis exhibited the ability to perform MICP, utilizing various calcium salts in the following order: chloride > acetate > nitrate > citrate. However, acetate is the most efficient salt of calcium to be converted to calcium carbonate precipitate by B. subtilis, as it exhibited the highest conversion efficiency percentage (g/g %), with the least cost and highest economic feasibility. Carbon dioxide removal (CDR) occurs at simultaneous equity to CaCO3 precipitation at mole/mole ratios. Economic feasibility (US$/m3) showed that BICCP may be applicable in CDR for cleansing carbon dioxide inside closed systems and for environmental safety. The bacterially induced CaCO3 proved successful applicability in improving the field capacity of sand soil and growth of V. faba, healing concrete cracks and sorption of heavy metals for depolluting sewage/wastewater for hygiene reuse. BICCP could repair concrete cracks of 1-2 mm wide in 7 days by 210 * 106 cells/mL. Adsorption of heavy metals (Pd, Zn, Cd and Cu) for partial removal of contaminants in/from waste/sewage water for hygiene reuse.
Keywords: Bacillus subtilis; BICCP; Calcium carbonate; Carbon capture; Crack healing; Mineral sorption; Nitrogenase; Urease; Water holding capacity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing of interests: The authors declare that they have no conflict of interest. Human and animal rights: Neither humans nor animals have been used in this study. Consent to participate: The authors agree to participate in this paper. Consent to publication: The authors agree to publish this paper in Nature ecology and evolution.
Figures


Similar articles
-
Bacillus megaterium favours CO₂ mineralization into CaCO₃ over the ureolytic pathway.Sci Rep. 2025 Jul 1;15(1):21861. doi: 10.1038/s41598-025-07323-9. Sci Rep. 2025. PMID: 40594585 Free PMC article.
-
Thermophiles in nanosized biocalcification: a novel approach for heavy metal remediation.Biometals. 2025 Aug;38(4):1203-1221. doi: 10.1007/s10534-025-00700-x. Epub 2025 May 24. Biometals. 2025. PMID: 40411706
-
Removal of high concentrations of zinc, cadmium, and nickel heavy metals by Bacillus and Comamonas through microbially induced carbonate precipitation.Biodegradation. 2025 May 5;36(3):40. doi: 10.1007/s10532-025-10131-7. Biodegradation. 2025. PMID: 40323541 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher's disease: a systematic review.Health Technol Assess. 2006 Jul;10(24):iii-iv, ix-136. doi: 10.3310/hta10240. Health Technol Assess. 2006. PMID: 16796930
References
-
- Aylward, G. & Findlay, T. SI Chemical Data Book 4th edn. (John Wiley, 2008).
-
- Bindschedler, S., Cailleau, G. & Verrecchia, E. Role of fungi in the biomineralization of calcite. Minerals6, 1–19 (2016).
-
- Gaëtan, J. et al. Widespread formation of intracellular calcium carbonates by the bloom-forming cyanobacterium Microcystis. Environ. Microbiol.25(3), 751–765 (2022). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

