Unraveling the effects of FLASH and conventional irradiation on retinal pigment epithelial cells: in vitro and in vivo studies
- PMID: 40594566
- PMCID: PMC12218338
- DOI: 10.1038/s41598-025-06101-x
Unraveling the effects of FLASH and conventional irradiation on retinal pigment epithelial cells: in vitro and in vivo studies
Abstract
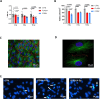
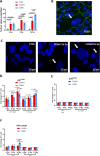
The retinal pigment epithelium (RPE) is a fundamental monolayer of pigmented cells that supports visual function, situated between the neural retina and choroidal blood vessels. Radiotherapy is a common treatment for ocular tumors such as uveal melanoma; however, the RPE is inevitably exposed to radiation during treatment. FLASH radiotherapy, characterized by ultra-high dose-rates, has emerged as a promising approach to minimize normal tissue toxicity while maintaining tumor control. Despite its potential, few preclinical studies have explored the effects of FLASH irradiation on the RPE, and no studies have directly compared FLASH with conventional (CONV) radiotherapy in this context. Using a LINAC capable of switching between FLASH and CONV irradiation, we here address the radiobiological effects on ARPE-19 cells, an in vitro RPE model, and the RPE of living mice. FLASH treatment demonstrated protective effects on ARPE-19 cells, enhancing cell viability and modulating cytokine expression (e.g., IL-6, IL-8). Furthermore, RPE tissue exhibited dose-dependent radiation sensitivity, with FLASH-irradiated mice performing better than CONV-treated ones. These findings indicate that FLASH radiotherapy may protect the RPE during ocular tumor treatment and provide insights into tissue-specific radiation sensitivity in the eye, supporting further research into its clinical applications.
Keywords: FLASH radiotherapy; Linear accelerator (LINAC); Ocular tumors; Radiation-induced toxicity; Retinal pigment epithelium (RPE); Uveal melanoma.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

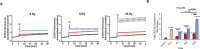



Similar articles
-
Peripheral iridotomy for pigmentary glaucoma.Cochrane Database Syst Rev. 2016 Feb 12;2(2):CD005655. doi: 10.1002/14651858.CD005655.pub2. Cochrane Database Syst Rev. 2016. PMID: 26871761 Free PMC article.
-
Dosimetric calibration of anatomy-specific ultra-high dose rate electron irradiation platform for preclinical FLASH radiobiology experiments.Med Phys. 2024 Dec;51(12):9166-9178. doi: 10.1002/mp.17432. Epub 2024 Sep 27. Med Phys. 2024. PMID: 39331834 Free PMC article.
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
-
The influence of femtosecond laser intrastromal lenticules on the characteristics and maturity in tissue-engineered stem cell-derived retinal pigment epithelium sheets.Stem Cell Res Ther. 2025 Jun 20;16(1):316. doi: 10.1186/s13287-025-04463-7. Stem Cell Res Ther. 2025. PMID: 40542390 Free PMC article.
-
Evaluation of Gene Expression and the Regulatory Role of microRNAs Related to the Mitogen-Activated Protein Kinase Signaling Pathway in Human Retinal Pigment Epithelial Cells Treated With Lipopolysaccharide A and Tacrolimus.Mediators Inflamm. 2025 Jul 1;2025:8586711. doi: 10.1155/mi/8586711. eCollection 2025. Mediators Inflamm. 2025. PMID: 40630080 Free PMC article.
References
-
- Chaikh, A. et al. Towards clinical application of ultra-high dose rate radiotherapy and the FLASH effect: challenges and current status. Cancer/Radiothérapie28, 463–473 (2024). - PubMed
-
- Horst, F. et al. Dose and dose rate dependence of the tissue sparing effect at ultra-high dose rate studied for proton and electron beams using the zebrafish embryo model. Radiother. Oncol.194, 110197 (2024). - PubMed
-
- COLLABORATIVEOCULARMELANOMASTU. The collaborative ocular melanoma study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma: IV. Ten-year mortality findings and prognostic factors. COMS report number 24. Am. J. Ophthalmol.138, 936–951 (2004). - PubMed
MeSH terms
Substances
Grants and funding
- Allergan contribution to research to ES/Allergan (an AbbVie Company)
- Project no. ECS_00000017; CUP I53C22000780001/EU funding within the Next Generation EU, Piano Nazionale di Ripresa e Resilienza (PNRR), Mission 4, Component 2, Innovation Ecosystems-Tuscany Health Ecosystem (THE), spoke 1 "Advanced Radiotherapies and Diagnostics in Oncology" to MC, G Sansevero, BD, FP, AC, MC, AG, SC, GG. and spoke 8 "Imaging in Neuroscience" to ES, BDM.
- INFN-FRIDA grant to to M C, BD, ES, FDM/INFN-FRIDA
- grant prog.n.134/2021 to CPFR/Fondazione Pisa
- #IG 2024 ID-30434 AIRC project to MC/Fondazione AIRC per la ricerca sul cancro ETS
LinkOut - more resources
Full Text Sources

