The use of pancreatic ductal adenocarcinoma 2D and 3D models to evaluate NDV infection, replication and induced cell death
- PMID: 40594584
- PMCID: PMC12219713
- DOI: 10.1038/s41598-025-06023-8
The use of pancreatic ductal adenocarcinoma 2D and 3D models to evaluate NDV infection, replication and induced cell death
Abstract
The development and testing of cancer therapies, such as oncolytic viro-immunotherapy, starts with 2-dimensional cell culture models, such as monolayers from lab-adapted cell lines. Although 2D models have been valuable, 3-dimensional models such as spheroids and patient-derived organoids (PDOs) better recapitulate tumor characteristics and may have higher predictive value for oncolytic viro-immunotherapy. Evaluating monolayers, spheroids, and PDOs for their response to oncolytic viro-immunotherapy using Newcastle Disease Virus (NDV) as an example may improve understanding of how model choice impacts outcomes. Monolayers, spheroids, and PDOs of Pancreatic Ductal Adenocarcinoma (PDAC) origin were evaluated for their response to NDV by assessing infection, replication, and virus-induced cell death. In spheroids and dense PDOs, NDV mainly infected the outer cell layer and did not spread to the inner layers. Cystic PDOs vary in susceptibility to NDV infection, replication, and virus-induced cell death, likely due to differences in genetic makeup. Evaluation of PDAC monolayers, spheroids, and PDOs revealed differences in NDV-induced cell death. Spheroids and dense PDOs may be more suitable than monolayers for evaluating virus infection. PDOs, regardless of morphology, reflect patient tumor genetics and might be a better model to identify markers to OV-induced cell death, advancing personalized oncolytic viro-immunotherapy approaches.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: Fresh tumor samples were collected from patients with PDAC that were part of a previously described clinical study18. This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the local medical ethics committee (MEC-2015–085.
Figures
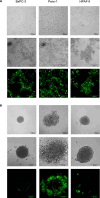
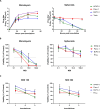
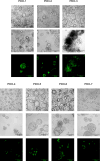
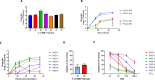
References
-
- Brancato, V., Oliveira, J. M., Correlo, V. M., Reis, R. L. & Kundu, S. C. Could 3D models of cancer enhance drug screening?. Biomaterials232(1), 1–14 (2020). - PubMed
MeSH terms
Grants and funding
- #16.01 and #19.10/Dutch Foundation OAK ("Overleven met Alvleesklier kanker") from the Netherlands
- #16.01 and #19.10/Dutch Foundation OAK ("Overleven met Alvleesklier kanker") from the Netherlands
- #15414/NWO-domein Toegepaste en Technische Wetenschappen
- #15414/NWO-domein Toegepaste en Technische Wetenschappen
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

