Toll like receptor 2 mediated exacerbation of sepsis associated acute kidney injury by renal congestion in mice
- PMID: 40594598
- PMCID: PMC12216951
- DOI: 10.1038/s41598-025-05878-1
Toll like receptor 2 mediated exacerbation of sepsis associated acute kidney injury by renal congestion in mice
Abstract
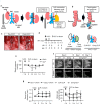
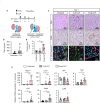
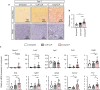
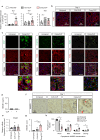
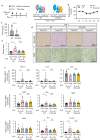
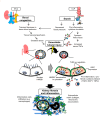
Renal congestion is a key factor in renal dysfunction associated with heart failure. We previously reported that renal congestion worsened renal ischemia-reperfusion in a murine model. However, its impact on sepsis-associated acute kidney injury (SA-AKI), the leading cause of AKI, remains unclear. Therefore, we herein investigated the mechanisms by which renal congestion exacerbates SA-AKI, with a focus on Toll-like receptor (TLR) 2. After inducing sepsis with cecal ligation and puncture (CLP) in a unilateral renal congestion model, transient blood pressure reductions and persistent renal vein dilation were observed. A histological analysis showed increased fibrosis and its markers in congested kidneys post-CLP. Acute phase results revealed extensive tubular damage, macrophage infiltration, TLR2 up-regulation, and elevated high mobility group box 1 (HMGB1) levels. In TLR2-knockout mice, exacerbation of tissue fibrosis by renal congestion was attenuated after CLP. In vitro, oxidative stress and hypoxia up-regulated TLR2 expression. Collectively, these results suggest that renal congestion and sepsis synergistically worsened renal damage, likely through hypoxia and the oxidative stress-induced activation of the TLR2 pathway.
Keywords: Acute kidney injury; Renal congestion; Sepsis; Toll-like receptor 2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- Junho, C. V. C. et al. Cardiorenal syndrome: Long road between kidney and heart. Heart Fail. Rev.27, 2137–2153. 10.1007/s10741-022-10218-w (2022). - PubMed
-
- Damman, K. et al. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J. Am. Coll. Cardiol.53, 582–588. 10.1016/j.jacc.2008.08.080 (2009). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

