Screening of electrospun PS/PCL scaffolds for three-dimensional triple negative breast cancer cell culture: impact of solvent, hydrophobicity, and setup orientation
- PMID: 40594674
- PMCID: PMC12219171
- DOI: 10.1038/s41598-025-05871-8
Screening of electrospun PS/PCL scaffolds for three-dimensional triple negative breast cancer cell culture: impact of solvent, hydrophobicity, and setup orientation
Abstract
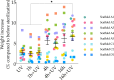
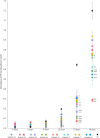
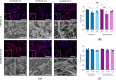
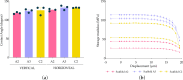
Triple-Negative Breast Cancer (TNBC) presents a significant challenge due to its aggressiveness and lack of targeted therapies. Understanding the interaction between TNBC cells and the extracellular matrix (ECM) in three-dimensional (3D) culture systems is vital for developing accurate in vitro models. This study explores the impact of electrospinning setup orientation, solvent selection, and polymer composition on scaffold design and TNBC cell culture. Various polystyrene (PS) and poly-ε-caprolactone (PCL) combinations were electrospun using different solvent combinations (dichloromethane/dimethylformamide (DCM/DMF), tetrahydrofuran/dimethylformamide (THF/DMF), chloroform/dichloromethane (Chl/DCM) and acetone) and setup orientations (vertical, horizontal). Scaffolds were characterized using Scanning Electron Microscopy (SEM) to assess fiber diameter and pore size. Cell proliferation and morphology were analyzed through MTT assay, SEM and Confocal Laser Scanning Microscopy (CLSM). Pore area and fiber diameter were influenced by solvent combination (THF/DMF < DCM/DMF < acetone < Chl/DCM) and orientation setup (horizontal < vertical). Sterilization assay revealed that 1-hour immersion in 70% ethanol followed by 30 min ultra-violet (UV) light exposure achieved sterilization with minimal scaffold degradation. High proliferation with no significant reduction compared to monolayer culture was found in some scaffolds and variability in cell morphology between scaffolds was also detected. Results highlight the critical role of scaffold printing parameters for 3D TNBC cell culture. Electrospun PS/PCL 40/60 scaffolds dissolved in DCM/DMF are promising in vitro models, providing a valuable tool for cancer research.
Keywords: Electrospinning; Fiber morphology; Poly-ε-caprolactone; Polystyrene; Scaffolds; Three-dimensional cell culture.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Cancer Today. World Health Organization (WHO). Global Cancer Observatory (GLOBOCAN) belbel.
MeSH terms
Substances
Grants and funding
- PI19/00372/Fundación Ramón Areces, Instituto de Salud Carlos III and co-funded by European Union (ERDF/ESF, "A way to make Europe"/"Investing in your future")
- PI19/00372/Fundación Ramón Areces, Instituto de Salud Carlos III and co-funded by European Union (ERDF/ESF, "A way to make Europe"/"Investing in your future")
- PI19/00372/Fundación Ramón Areces, Instituto de Salud Carlos III and co-funded by European Union (ERDF/ESF, "A way to make Europe"/"Investing in your future")
- PI19/00372/Fundación Ramón Areces, Instituto de Salud Carlos III and co-funded by European Union (ERDF/ESF, "A way to make Europe"/"Investing in your future")
- PI19/00372/Fundación Ramón Areces, Instituto de Salud Carlos III and co-funded by European Union (ERDF/ESF, "A way to make Europe"/"Investing in your future")
- PLEC2021-007523/AEI/10.13039/501100011033/Agencia Estatal de Investigación (Spain) and European Union NextGenerationEU
- PLEC2021-007523/AEI/10.13039/501100011033/Agencia Estatal de Investigación (Spain) and European Union NextGenerationEU
- PLEC2021-007523/AEI/10.13039/501100011033/Agencia Estatal de Investigación (Spain) and European Union NextGenerationEU
- PLEC2021-007523/AEI/10.13039/501100011033/Agencia Estatal de Investigación (Spain) and European Union NextGenerationEU
- PLEC2021-007523/AEI/10.13039/501100011033/Agencia Estatal de Investigación (Spain) and European Union NextGenerationEU
LinkOut - more resources
Full Text Sources

