Artificial intelligence derived grading of mustard gas induced corneal injury and opacity
- PMID: 40594758
- PMCID: PMC12216909
- DOI: 10.1038/s41598-025-08042-x
Artificial intelligence derived grading of mustard gas induced corneal injury and opacity
Abstract
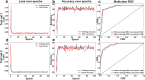
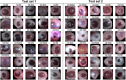
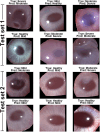
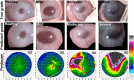
Artificial intelligence (AI) has emerged as a transformative tool in ophthalmology for disease diagnosis and prognosis. However, use of AI for assessing corneal damage due to chemical injury in live rabbits remains lacking. This study aimed to develop an AI-derived clinical classification model for an objective grading of corneal injury and opacity levels in live rabbits following ocular exposure of sulfur mustard (SM). An automated method to grade corneal injury minimizes diagnostic errors and enhances translational application of preclinical research in better human eyecare. SM induced corneal injury and opacity from 401 in-house rabbit corneal images captured with a clinical stereomicroscope were used. Three independent subject matter specialists classified corneal images into four health grades: healthy, mild, moderate, and severe. Mask-RCNN was employed for precise corneal segmentation and extraction, followed by classification using baseline convolutional neural network and transfer learning algorithms, including VGG16, ResNet101, DenseNet121, InceptionV3, and ResNet50. The ResNet50-based model demonstrated the best performance, achieving 87% training accuracy, and 85% and 83% prediction accuracies on two independent test sets. This deep learning framework, combining Mask-RCNN with ResNet50 allows reliable and uniform grading of SM-induced corneal injury and opacity levels in affected eyes.
Keywords: Artificial intelligence; Cornea; Fibrosis; Pathology; Sulfur mustard.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Disclaimer: The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Figures


Similar articles
-
Artificial intelligence for detecting keratoconus.Cochrane Database Syst Rev. 2023 Nov 15;11(11):CD014911. doi: 10.1002/14651858.CD014911.pub2. Cochrane Database Syst Rev. 2023. PMID: 37965960 Free PMC article.
-
A deep learning approach to direct immunofluorescence pattern recognition in autoimmune bullous diseases.Br J Dermatol. 2024 Jul 16;191(2):261-266. doi: 10.1093/bjd/ljae142. Br J Dermatol. 2024. PMID: 38581445
-
Accuracy of artificial intelligence model for infectious keratitis classification: a systematic review and meta-analysis.Front Public Health. 2023 Nov 24;11:1239231. doi: 10.3389/fpubh.2023.1239231. eCollection 2023. Front Public Health. 2023. PMID: 38074720 Free PMC article.
-
A practical and safer model of nitrogen mustard injury in cornea.PLoS One. 2025 Jul 3;20(7):e0327622. doi: 10.1371/journal.pone.0327622. eCollection 2025. PLoS One. 2025. PMID: 40608742 Free PMC article.
-
Artificial Intelligence-Based prediction model for surgical site infection in metastatic spinal disease: a multicenter development and validation study.Int J Surg. 2025 Jun 27. doi: 10.1097/JS9.0000000000002806. Online ahead of print. Int J Surg. 2025. PMID: 40576176
References
-
- Quan, X. et al. SCINet: A segmentation and classification interaction CNN method for arteriosclerotic retinopathy grading. Interdiscip. Sci.16 (4), 926–935. 10.1007/s12539-024-00650-x2024 (2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

