Multiomics approach provides insight into altered choline metabolism and liver injury in patients with glycogen storage disease type Ia
- PMID: 40594807
- PMCID: PMC12216020
- DOI: 10.1038/s41598-025-06272-7
Multiomics approach provides insight into altered choline metabolism and liver injury in patients with glycogen storage disease type Ia
Abstract
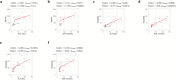
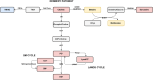
Glycogen storage disease type Ia (GSDIa) is an inherited disorder of carbohydrate metabolism. Patients present with excessive storage of glycogen and fat in the liver and kidneys and are potentially at risk of developing long-term complications. Currently, the mainstay of treatment is highly tailored dietary regimens aimed at improving metabolic control. In the present study, to better elucidate the mechanisms potentially involved in the development of long-term complications, a mass spectrometry-based strategy was employed for an in-depth characterization of the serum proteomic and metabolomic profile of n.12 GSDIa patients. The detection of differential abundance of highly liver-specific circulating proteins and choline-related metabolites in patients provides new insights into the extent of liver damage and dysregulation of lipid metabolism in GSDIa. Specifically, the differential abundance of serum aldolase B and its positive correlation with traditional liver function markers supports its role as a potential biomarker for long-term monitoring of GSDIa liver injury.
Keywords: ALDOB; Glycogen storage disease type Ia; Lipid metabolism; Liver injury; Multiomics; Serum biomarkers.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Clinical characteristics of 50 hepatocellular adenoma patients among 164 cases of glycogen storage disease type Ia.Eur J Pediatr. 2025 Jun 30;184(7):458. doi: 10.1007/s00431-025-06180-y. Eur J Pediatr. 2025. PMID: 40586975
-
Multiomics reveals metformin's dual role in gut microbiome remodeling and hepatic metabolic reprogramming for MAFLD intervention.Sci Rep. 2025 Jul 2;15(1):22699. doi: 10.1038/s41598-025-07557-7. Sci Rep. 2025. PMID: 40594788 Free PMC article.
-
Understanding Glycogen Storage Disease Type IX: A Systematic Review with Clinical Focus-Why It Is Not Benign and Requires Vigilance.Genes (Basel). 2025 May 15;16(5):584. doi: 10.3390/genes16050584. Genes (Basel). 2025. PMID: 40428406 Free PMC article. Review.
-
Integrated multi-omics analysis reveals the functional signature of microbes and metabolomics in pre-diabetes individuals.Microbiol Spectr. 2025 Jul;13(7):e0145924. doi: 10.1128/spectrum.01459-24. Epub 2025 Jun 9. Microbiol Spectr. 2025. PMID: 40488467 Free PMC article.
-
Risk prediction of hepatotoxicity in paracetamol poisoning.Clin Toxicol (Phila). 2017 Sep;55(8):879-892. doi: 10.1080/15563650.2017.1317349. Epub 2017 Apr 27. Clin Toxicol (Phila). 2017. PMID: 28447858
References
-
- Hannah, W. B. et al. Glycogen storage diseases. Nat. Rev. Dis. Primers9, 46. 10.1038/s41572-023-00456-z (2023). - PubMed
-
- Chou, J. Y., Jun, H. S. & Mansfield, B. C. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase/glucose-6-phosphate transporter complexes. J. Inherit. Metab. Dis.38, 511–519 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

