Higher dopamine D1 receptor expression in prefrontal parvalbumin neurons underlies higher distractibility in marmosets versus macaques
- PMID: 40594842
- PMCID: PMC12214923
- DOI: 10.1038/s42003-025-08297-0
Higher dopamine D1 receptor expression in prefrontal parvalbumin neurons underlies higher distractibility in marmosets versus macaques
Abstract
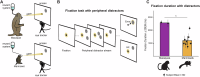
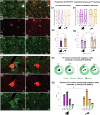
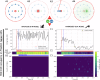
Marmosets and macaques are common nonhuman primate models of cognition, yet marmosets appear more distractible and perform worse in cognitive tasks. The dorsolateral prefrontal cortex (dlPFC) is pivotal for sustained attention, and research in macaques suggests that dopaminergic modulation and inhibitory parvalbumin (PV) neurons could influence distractor resistance. Here we compare the two species using a visual fixation task with distractors, perform molecular and anatomical analyses in dlPFC, and link functional microcircuitry with cognitive performance using computational modeling. We show that marmosets are more distractible than macaques, and that marmoset dlPFC PV neurons contain higher levels of dopamine D1 receptor (D1R) transcripts and protein, similar to levels in mice. Our modeling indicates that higher D1R expression in marmoset dlPFC PV neurons may increase distractibility by making dlPFC microcircuits more vulnerable to disruptions of their task-related persistent activity, especially when dopamine is released in dlPFC in response to unexpected salient stimuli.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
Peripuberty Is a Sensitive Period for Prefrontal Parvalbumin Interneuron Activity to Impact Adult Cognitive Flexibility.Dev Neurosci. 2025;47(2):127-138. doi: 10.1159/000539584. Epub 2024 Jun 3. Dev Neurosci. 2025. PMID: 38830346 Free PMC article.
-
Timing of Methamphetamine Exposure during Adolescence Differentially Influences Parvalbumin and Perineuronal Net Immunoreactivity in the Medial Prefrontal Cortex of Female, but Not Male, Rats.Dev Neurosci. 2025;47(1):27-39. doi: 10.1159/000538608. Epub 2024 Mar 28. Dev Neurosci. 2025. PMID: 38547851 Free PMC article.
-
Dopamine D1 receptor in medial prefrontal cortex mediates the effects of TAAR1 activation on chronic stress-induced cognitive and social deficits.Neuropsychopharmacology. 2024 Jul;49(8):1341-1351. doi: 10.1038/s41386-024-01866-7. Epub 2024 Apr 24. Neuropsychopharmacology. 2024. PMID: 38658737 Free PMC article.
-
Antiemetics for adults for prevention of nausea and vomiting caused by moderately or highly emetogenic chemotherapy: a network meta-analysis.Cochrane Database Syst Rev. 2021 Nov 16;11(11):CD012775. doi: 10.1002/14651858.CD012775.pub2. Cochrane Database Syst Rev. 2021. PMID: 34784425 Free PMC article.
-
A Systematic Review and Meta-Analysis of the Effects of Transcranial Direct Current Stimulation (tDCS) Over the Dorsolateral Prefrontal Cortex in Healthy and Neuropsychiatric Samples: Influence of Stimulation Parameters.Brain Stimul. 2016 Jul-Aug;9(4):501-17. doi: 10.1016/j.brs.2016.04.006. Epub 2016 Apr 12. Brain Stimul. 2016. PMID: 27160468
Cited by
-
From comparative connectomics to large-scale working memory modeling in macaque and marmoset.bioRxiv [Preprint]. 2025 Mar 17:2025.03.17.643781. doi: 10.1101/2025.03.17.643781. bioRxiv. 2025. PMID: 40166341 Free PMC article. Preprint.
References
-
- Easton, A., Parker, K., Derrington, A. M. & Parker, A. Behaviour of marmoset monkeys in a T-maze: comparison with rats and macaque monkeys on a spatial delayed non-match to sample task. Exp. Brain Res.150, 114–116 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

