Dual treatment with Val-083 and AZD1775 shows potent anti-tumor activity in diffuse midline glioma models
- PMID: 40594881
- PMCID: PMC12215462
- DOI: 10.1038/s41698-025-01006-4
Dual treatment with Val-083 and AZD1775 shows potent anti-tumor activity in diffuse midline glioma models
Abstract
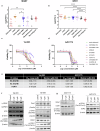
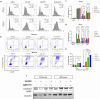
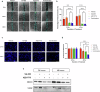
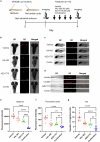
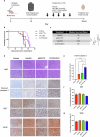
H3K27M diffuse midline gliomas (DMG) are characterized by p53 mutations and hypomethylation of MGMT, a DNA-repair enzyme, leading to resistance towards chemotherapeutic agents such as temozolomide (TMZ). As an alternative, we investigated the efficacy of a functionally different DNA-damaging agent, Val-083, on our DMG models. Val-083 is a blood-brain barrier penetrant DNA targeting agent that induces DNA N7-guanine interstrand crosslinks, which is unrepairable by MGMT. As Val-083 also triggers S/G2 phase cell cycle arrest for DNA repair, we evaluated its combined efficacy with Wee1 inhibitor, AZD1775. AZD1775 functions by inhibiting Wee1, at G2/M checkpoint to prevent phosphorylation of CDK1 and propel cells into the M phase. This subsequently overrides cell cycle arrest and drives cells with DNA damage into premature mitosis and apoptosis. Our results showed that Val-083 and AZD1775 work additively on a range of p53 mutant and p53 wildtype DMG models to inhibit cell growth, induce DNA damage and alter cell cycle. In addition, the combination drugs led to significant increase in the number of cells undergoing apoptosis, and a decrease in the migration and invasion activity of the cells. In vivo, the combination of both drugs led to significant reduction in tumor growth in zebrafish xenograft models and prolongation of survival in mice xenograft models. Our findings indicate that Val-083 and AZD1775 in combination demonstrate promising efficacy in DMGs, providing a clinical rationale for positioning these arms in future therapies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Hoffman, L. M. et al. Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (DIPG): a collaborative report from the international and european society for pediatric oncology dipg registries. J. Clin. Oncol.36, 1963–72 (2018). - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

