Preparation and properties study of self-healing anticorrosive coating based on MCM-41 nanocontainer and triazinyl corrosion inhibitor
- PMID: 40594986
- PMCID: PMC12215977
- DOI: 10.1038/s41598-025-06528-2
Preparation and properties study of self-healing anticorrosive coating based on MCM-41 nanocontainer and triazinyl corrosion inhibitor
Abstract
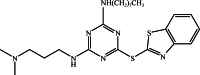
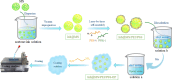
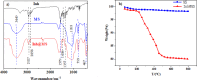
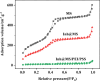
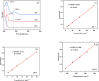
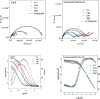
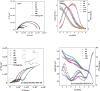
In this paper, a self-healing anticorrosive coating with pH response based on epoxy was prepared. Firstly, triazinyl corrosion inhibitor (Inh) was loaded into mesoporous molecular sieve (MCM-41) by the vacuum impregnation method. Polyethylenimine (PEI) and sodium polystyrene sulfonate (PSS) were coated on the surface through the layer-by-layer (LBL) self-assembly method. Then a pH-responsive self-healing anti-corrosion coating Inh@MS/PEI/PSS-EP was obtained by adding the newly prepared nanocapsules to the organic epoxy coating. Eventually, foutier transform infrared spectroscopy (FT-IR), N2 adsorption/desorption, Zeta potential test, thermogravimetry (TG), testultraviolet-visible absorption spectroscopy (UV-vis), electrochemical impedance spectroscopy (EIS), scanning electron microscopy (SEM), and atomic force microscopy (AFM) were used to investigate the pH response, corrosion resistance, self-healing properties of the prepared coating. The results demonstrate that the coating prepared by this method can actively release Inh in alkaline environment. EIS and surface topography analysis manifest the decay-resistance of the organic epoxy coating was improved. The results of the molecular dynamics (MD) simulation show that Inh can adsorb on the carbon steel surface, which is consistent with the experimental results. The corrosion resistance of the modified epoxy coating was improved, after 30 days of immersion, the |Z|0.01 Hz of the modified epoxy coating is 3 orders of magnitude higher than that of the pure epoxy coating.
Keywords: Mesoporous molecular sieve; Polyelectrolyte; Self-healing coating; pH response.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures








References
-
- Cen, H. Y. et al. Functionalized carbon nanotubes as a novel inhibitor to enhance the anticorrosion performance of carbon steel in CO2-saturated NaCl solution. Corros. Sci.177, 109011. 10.1016/j.corsci.2020.109011 (2020). - DOI
-
- Ye, Y. W. et al. Superhydrophobic oligoaniline-containing electroactive silica coating as pre-process coating for corrosion protection of carbon steel. Chem. Eng. J.348, 940–951. 10.1016/j.cej.2018.02.053 (2018). - DOI
-
- Ana, S. V. et al. Properties of hybrid sol-gel coatings with the incorporation of lanthanum 4-hydroxy cinnamate as corrosion inhibitor on carbon steel with different surface finishes. Appl. Surf. Sci.561, 149881. 10.1016/j.apsusc.2021.149881 (2021). - DOI
-
- Zhang, M. X. et al. Low-cost preparation of durable, transparent, superhydrophobic coatings with excellent environmental stability and self-cleaning function. Surf. Coat. Technol.438, 128367. 10.1016/j.surfcoat.2022.128367 (2022). - DOI
-
- Zhang, Y. et al. Excellent corrosion protection performance of epoxy composite coatings filled with silane functionalized silicon nitride. J. Polym. Res.25(5), 130. 10.1007/s10965-018-1518-2 (2018). - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

