Use of bovine serum albumin might impair immunofluorescence signal in thick tissue samples
- PMID: 40594992
- PMCID: PMC12215187
- DOI: 10.1038/s41598-025-06876-z
Use of bovine serum albumin might impair immunofluorescence signal in thick tissue samples
Abstract
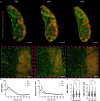
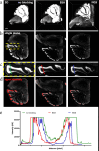
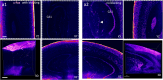
Significant progress in microscopic imaging techniques allowed transition from predominantly qualitative methods to a powerful tool for quantitative research, driven by improved instrumentation and computational power. Furthermore, previously limited to thin, laser-permeable tissue sections, imaging techniques have been revolutionized by the advent of tissue optical clearing. This innovation enables the visualization and quantitative analysis of entire organs and even whole bodies at cellular resolution. However, achieving high-quality imaging depends not only on the transparency of the tissue preparation but also on precise immunofluorescence labeling to ensure accurate signal detection and reliable study outcomes. In this study, we evaluated whether various reagents that are typically applied during the tissue blocking step prior to immunofluorescence staining affect the quality of the obtained image in thick and optically cleared samples. We demonstrate that the commonly employed tissue blocking step does not improve imaging conditions and even can substantially degrade fluorescence signal quality, particularly in large, optically cleared tissues such as whole mouse brain hemispheres.
Keywords: BSA; Bovine serum albumin; Confocal microscopy; Immunofluorescence; Tissue clearing.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Electric fans for reducing adverse health impacts in heatwaves.Cochrane Database Syst Rev. 2012 Jul 11;2012(7):CD009888. doi: 10.1002/14651858.CD009888.pub2. Cochrane Database Syst Rev. 2012. PMID: 22786530 Free PMC article.
-
Novel application of metabolic imaging of early embryos using a light-sheet on-a-chip device: a proof-of-concept study.Hum Reprod. 2025 Jan 1;40(1):41-55. doi: 10.1093/humrep/deae249. Hum Reprod. 2025. PMID: 39521726 Free PMC article.
-
Magnetic resonance perfusion for differentiating low-grade from high-grade gliomas at first presentation.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD011551. doi: 10.1002/14651858.CD011551.pub2. Cochrane Database Syst Rev. 2018. PMID: 29357120 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Validation of an organ mapping antibody panel for cyclical immunofluorescence microscopy on normal human kidneys.Am J Physiol Renal Physiol. 2024 Jul 1;327(1):F91-F102. doi: 10.1152/ajprenal.00426.2023. Epub 2024 May 9. Am J Physiol Renal Physiol. 2024. PMID: 38721662 Free PMC article.
References
-
- Susaki, E. A. et al. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nat. Protoc.10, 1709–1727 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

