Modulation of Bifidobacterium by HD5 during weaning is associated with high abundance in later life
- PMID: 40595013
- PMCID: PMC12219304
- DOI: 10.1038/s43856-025-00977-6
Modulation of Bifidobacterium by HD5 during weaning is associated with high abundance in later life
Abstract
Background: Bifidobacterium colonization of the intestine is believed to have beneficial effects on our health from infancy throughout life. However, how particular members of the genus Bifidobacterium colonize the neonatal intestine and whether early-life bifidobacterial colonization affects establishment of Bifidobacterium-rich microbiota in later life remain unanswered. α-Defensin secreted from small intestinal Paneth cells elicits selective bactericidal activities that efficiently kill pathogens while hardly affecting commensals including Bifidobacterium in vitro, thus contributing to intestinal microbiota regulation.
Methods: One hundred forty-eight fecal samples were serially obtained from 33 children from postnatal 3-5 days to 3 years old, conducting a longitudinal cohort study of mothers and children living in Iwamizawa city, Hokkaido, Japan (SMILE Iwamizawa study). Microbiota composition and secretory level of α-defensin, human defensin 5 (HD5), were assessed to investigate the relationship between HD5 and Bifidobacterium colonization.
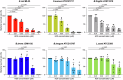
Results: We show that HD5 is associated with colonization of Bifidobacterium in early life from pre-weaning to weaning periods. Furthermore, high relative abundance of Bifidobacterium in the weaning period, which positively correlates with HD5 secretion, is associated with the establishment of Bifidobacterium-rich microbiota at 3 years old, when the intestinal microbiota matures.
Conclusions: This study suggests the importance of the weaning period in establishing long-lasting homeostasis interwoven with the host innate immunity and Bifidobacterium in the intestinal microbiota.
Plain language summary
Bifidobacterium is a beneficial bacterium that lives in the intestine and supports our health from infancy to adulthood. However, it is not fully understood how these bacteria first colonize the babies’ intestine, or whether this early colonization affects the intestinal environment in later life. We followed 33 children from 3–5 days to 3 years old and collected fecal samples to study their intestinal bacteria. We also measured an antimicrobial peptide called HD5, which is known to control the intestinal bacteria. We found that HD5 modulates the colonization of Bifidobacterium, and that a high level of these bacteria during weaning may help establish a healthy intestinal environment that lasts. These findings suggest that supporting intestinal health during weaning benefit long-term well-being.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Y.S., H.I., S.K., F.T., and Y.T. are employees of Morinaga Milk Industry Co., Ltd. Other authors (Y.Y., S.O., M.I., T.K., Koshi N., A.T., T.A., Kiminori N.) declare no competing interests. Ethics approval: This study was approved by the ethics committees of the Graduate School of Medicine, Hokkaido University (16-039) and Morinaga Milk Industry Co., Ltd (16005-144).
Figures





References
-
- United Nations Children’s Fund (UNICEF). Improving Child Nutrition: The achievable imperative for global progress. https://www.unicef.or.jp/library/pdf/Nutrition_Report_20130416.pdf (2013).
Grants and funding
- JPMJCE 1301/MEXT | Japan Science and Technology Agency (JST)
- JPMJPF2108/MEXT | Japan Science and Technology Agency (JST)
- 21H02891/MEXT | Japan Society for the Promotion of Science (JSPS)
- 20H04098/MEXT | Japan Society for the Promotion of Science (JSPS)
- 22K19120/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources
Medical

