DunedinPACNI estimates the longitudinal Pace of Aging from a single brain image to track health and disease
- PMID: 40595015
- PMCID: PMC12350157
- DOI: 10.1038/s43587-025-00897-z
DunedinPACNI estimates the longitudinal Pace of Aging from a single brain image to track health and disease
Abstract
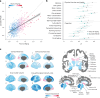
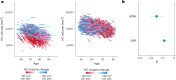
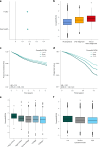
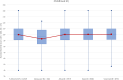
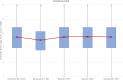
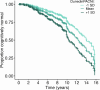
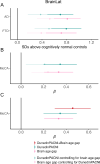
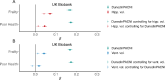
To understand how aging affects functional decline and increases disease risk, it is necessary to develop measures of how fast a person is aging. Using data from the Dunedin Study, we introduce an accurate and reliable measure for the rate of longitudinal aging derived from cross-sectional brain magnetic resonance imaging, that is, the Dunedin Pace of Aging Calculated from NeuroImaging (DunedinPACNI). Exporting this measure to the Alzheimer's Disease Neuroimaging Initiative, UK Biobank and BrainLat datasets revealed that faster DunedinPACNI predicted cognitive impairment, accelerated brain atrophy and conversion to diagnosed dementia. Faster DunedinPACNI also predicted physical frailty, poor health, future chronic diseases and mortality in older adults. When compared to brain age gap, DunedinPACNI was similarly or more strongly related to clinical outcomes. DunedinPACNI is a next-generation brain magnetic resonance imaging biomarker that can help researchers explore aging effects on health outcomes and evaluate the effectiveness of antiaging strategies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: K.S., A.C. and T.E.M. are listed as inventors of DunedinPACE, a Duke University and University of Otago invention licensed to TruDiagnostic for commercial uses; however, the DunedinPACE algorithm is open access for research purposes. The other authors declare no competing interests.
Figures







References
MeSH terms
Grants and funding
- P2C HD065563/HD/NICHD NIH HHS/United States
- R01 AG049789/AG/NIA NIH HHS/United States
- R01 AG073207/AG/NIA NIH HHS/United States
- R01AG032282/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- MR/X021149/1/RCUK | Medical Research Council (MRC)
- R01AG049789/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01AG073207/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- F99 AG068432/AG/NIA NIH HHS/United States
- R01 AG032282/AG/NIA NIH HHS/United States
- U19 AG024904/AG/NIA NIH HHS/United States
- K00 AG068432/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

