Direct recording of electrically evoked cortical potentials from cochlear implants demonstrates feasibility and clinical relevance in pediatric users
- PMID: 40595118
- PMCID: PMC12217970
- DOI: 10.1038/s41598-025-06652-z
Direct recording of electrically evoked cortical potentials from cochlear implants demonstrates feasibility and clinical relevance in pediatric users
Abstract
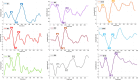
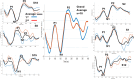
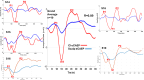
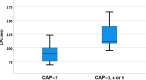
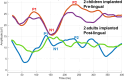
Intracochlear electrodes in cochlear implants (CIs) offer a novel method for recording auditory brain activity without external EEG equipment, addressing challenges in pediatric CI users. This study tested the feasibility of recording electrically evoked cortical auditory potentials (eCAEPs) directly via the CI system. Twenty children and three adults with bilateral Advanced Bionics CIs participated. A brief electrical stimulus was delivered to one CI, while the contralateral CI recorded responses using a basal electrode referenced to the case. Each session included stimulus and non-stimulus sweeps, with averaging over 600 ms revealing clear eCAEP patterns. All participants exhibited obligatory P1, N1, and P2 peaks within a test duration of under five minutes. The method showed good test-retest repeatability and expected latency shifts occurred with stimulus level adjustments. Compared to scalp recorded EEG, intracochlear recordings produced significantly larger amplitudes with similar latencies. Early implanted children displayed distinct eCAEP patterns, and better performing CI users had earlier P1 responses. This recording approach provides a robust, non-invasive tool for monitoring CI users, particularly young children, offering potential advancements in post-implantation assessment and intervention by eliminating external equipment while ensuring reliable recordings.
Keywords: Brain objective measure; Cortical auditory evoked potentials; Electrical stimulation; Intra-cochlear recordings; Pediatric; Scalp recordings.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures








Similar articles
-
Validation of direct recording of electrically evoked cortical auditory evoked potentials through a cochlear implant system.Sci Rep. 2024 Nov 17;14(1):28366. doi: 10.1038/s41598-024-79528-3. Sci Rep. 2024. PMID: 39551893 Free PMC article.
-
Cortical Auditory Evoked Potentials Recorded Directly Through the Cochlear Implant in Cochlear Implant Recipients: a Feasibility Study.Ear Hear. 2022 Sep-Oct 01;43(5):1426-1436. doi: 10.1097/AUD.0000000000001212. Epub 2022 Mar 3. Ear Hear. 2022. PMID: 35245922
-
Intraoperative Compound Action Potentials as a Predictor of Postoperative Cortical Auditory Evoked Potentials in Cochlear Implant Users.Audiol Neurootol. 2025;30(1):58-69. doi: 10.1159/000540576. Epub 2024 Jul 31. Audiol Neurootol. 2025. PMID: 39084205 Free PMC article.
-
The effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults: a systematic review and economic model.Health Technol Assess. 2009 Sep;13(44):1-330. doi: 10.3310/hta13440. Health Technol Assess. 2009. PMID: 19799825
-
Systematic review of compound action potentials as predictors for cochlear implant performance.Laryngoscope. 2017 Feb;127(2):476-487. doi: 10.1002/lary.26154. Epub 2016 Nov 2. Laryngoscope. 2017. PMID: 27804133
References
-
- Kral, A., Dorman, M. F. & Wilson, B. S. Neuronal development of hearing and language: Cochlear implants and critical periods. Annu. Rev. Neurosci.42(1), 47–65 (2019). - PubMed
-
- Asghari, A. et al. Complications and outcomes of cochlear implantation in children younger than 12 months: A multicenter study. Int. J. Pediatr. Otorhinolaryngol.167, 111495 (2023). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

