Aspirin improves short and long term survival outcomes of patients with sepsis associated encephalopathy
- PMID: 40595183
- PMCID: PMC12215694
- DOI: 10.1038/s41598-025-08075-2
Aspirin improves short and long term survival outcomes of patients with sepsis associated encephalopathy
Abstract
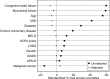
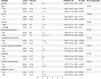
Sepsis-associated encephalopathy (SAE) is a common complication of sepsis, characterized by altered mental status and contributing to higher mortality. Aspirin, an antiplatelet agent with anti-inflammatory properties, may improve outcomes in patients with SAE. This study aims to evaluate the effect of aspirin on the prognosis of patients with SAE. A retrospective cohort study using the Medical Information Mart for Intensive Care IV (MIMIC-IV 2.2) database included 5840 patients with SAE: aspirin group (n = 3378) and non-aspirin group (n = 2462). Propensity score matching (PSM) at a 1:1 ratio resulted in 1770 matched pairs. The primary outcomes were 28-day, 90-day, 365-day, and 1095-day survival rates. Secondary outcomes included ICU length of stay, incidence of gastrointestinal bleeding, and thrombocytopenia. After PSM, baseline characteristics were balanced. The aspirin group had significantly higher survival rates at all time points (p < 0.05) compared to the non-aspirin group. ICU length of stay and incidence of gastrointestinal bleeding and thrombocytopenia were not significantly different between groups after matching. Subgroup analyses indicated that aspirin use was associated with improved 28-day survival in patients with SOFA scores ≥ 3, males, and those without chronic pulmonary disease or diabetes (all p < 0.05). Additionally, within the aspirin group, low-dose aspirin (81 mg/day) was associated with higher 365-day and 1095-day survival rates compared to the high-dose group (325 mg/day). Aspirin use in patients with SAE is associated with favorable short- and long-term survival outcomes without a significant increase in the risk of gastrointestinal bleeding or thrombocytopenia. Low-dose aspirin may provide potential long-term benefits. However, further prospective randomized controlled trials are needed to validate these findings.
Keywords: Aspirin; Prognosis; Retrospective cohort study; Sepsis-associated encephalopathy; Survival rate.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: The data in this study were from two public de-identified databases. After completing Collaborative Institutional Training Initiative (CITI program), we got permission to access the database (Record ID: 36067767). This study involves human participants but the Ethics Committee(s) or Institutional Board(s) exempted this study.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

