Extracellular vesicles derived from Lactobacillus gasseri GFC-1220 alleviate inflammation via the TLR4/NF-κB signaling pathway in LPS-stimulated RAW264.7 macrophages
- PMID: 40595215
- PMCID: PMC12215889
- DOI: 10.1038/s41598-025-99160-z
Extracellular vesicles derived from Lactobacillus gasseri GFC-1220 alleviate inflammation via the TLR4/NF-κB signaling pathway in LPS-stimulated RAW264.7 macrophages
Abstract
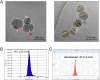
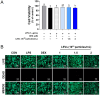
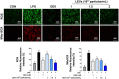
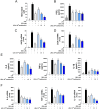
Studies on the impact of gram-positive extracellular vesicles have paved the way for novel medical advancements. These vesicles serve as efficient carriers of microbial molecules to target cells, thereby influencing human pathophysiological processes. Thus, this study aimed to investigate the anti-inflammatory properties of extracellular vesicles derived from Lactobacillus gasseri GFC-1220 (LEVs) in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages. LEVs were characterized using transmission electron microscopy, nanoparticle tracking analysis, and dynamic light scattering measurements to examine their morphology, size, and concentration. We further assessed the anti-inflammatory effects of LEVs and their underlying mechanism in LPS-stimulated RAW264.7 macrophages. Our findings revealed that LEVs did not cause cytotoxic effects and significantly decreased the level of mitochondria superoxide and reactive oxygen species production. In addition, these vesicles effectively inhibited the LPS-induced activation of TLR4/NF-κB signaling pathway, consequently suppressing the secretion and expression of various pro-inflammatory mediators and cytokines. These included a reduction in the production of NO and PGE2, along with their corresponding producer enzymes iNOS and COX-2, as well as IL-6, TNF-α, and IL-1β cytokines. These findings strongly suggest that LEVs have significant potential for the development of new anti-inflammatory agents.
Keywords: Lactobacillus gasseri GFC-1220; Anti-inflammatory effects; Extracellular vesicles; Macrophages.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Consent for publication: The authors declare that financial support was received for the research, authorship, and/or publication of this article.
Figures


Similar articles
-
Mahonia bealei (Fort.) Carr. Leaf extract modulates the TLR2/MyD88/NF-κB signaling pathway to inhibit PGN-induced inflammation in RAW264.7 cells.J Ethnopharmacol. 2025 Mar 26;344:119510. doi: 10.1016/j.jep.2025.119510. Epub 2025 Feb 17. J Ethnopharmacol. 2025. PMID: 39971016
-
Anti-inflammatory effects of Rehmannia glutinosa polysaccharide on LPS-induced acute liver injury in mice and related underlying mechanisms.J Ethnopharmacol. 2025 Jul 24;351:120099. doi: 10.1016/j.jep.2025.120099. Epub 2025 Jun 6. J Ethnopharmacol. 2025. PMID: 40484254
-
Mechanistic Insights into Isorhamnetin: Targeting MAPK and NF-κB Pathways to Mitigate LPS-Induced Inflammation.Curr Mol Pharmacol. 2024;17:e18761429385248. doi: 10.2174/0118761429385248250409060550. Curr Mol Pharmacol. 2024. PMID: 40296631
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

