CaM promotes cardiomyocyte mitophagy in myocardial ischemia-reperfusion injury involving in the regulation of the IP3R3-GRP75-VDAC1 complex
- PMID: 40595218
- PMCID: PMC12214813
- DOI: 10.1038/s41598-025-07977-5
CaM promotes cardiomyocyte mitophagy in myocardial ischemia-reperfusion injury involving in the regulation of the IP3R3-GRP75-VDAC1 complex
Abstract
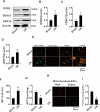
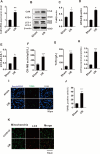
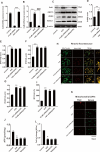
The pathogenesis of myocardial ischemia-reperfusion injury (MIRI) is not fully clear. This study aims to investigate the role of mitochondrial-associated endoplasmic reticulum membrane (MAM)-related calcium overload in mitophagy. In vitro and in vivo models were established to simulate MIRI. Cellular injury, apoptosis and mitophagy were measured and gene expression was analysized. The expression levels of glucose-regulated protein 75 (GRP75), receptor for inositol 1,4,5-trisphosphate (IP3R3), voltage-dependent anion-selective channel 1 (VDAC1), and calmodulin (CaM) and the mitochondrial calcium content, mitophagy and apoptosis were significantly increased in MIRI or hypoxia/reoxygenation (H/R) cells when compared to controls, but the mitochondrial membrane potential and ATP significantly decreased. GRP75 knockdown significantly inhibited CaM expression, mitochondrial calcium overload and mitophagy of H9C2 cells, whereas had no significant effect on IP3R3 and VDAC1 expression. CaM knockdown had no significant effect on the expression of GRP75, IP3R3 and VDAC1, and on mitochondrial calcium concentration, ATP levels and mitochondrial membrane potential of H9C2 cells, but significantly inhibited mitophagy and apoptosis. Collectively, these data suggest that the IP3R3-GRP75-VDAC1/CaM axis plays an important role in mitochondrial autophagy injury during myocardial ischemia-reperfusion and that it is a potential target for MIRI treatment.
Keywords: CaM; IP3R3-GRP75-VDAC1 complex; Mitochondrial calcium overload; Mitophagy; Myocardial ischemia reperfusion injury (MIRI).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: This study was approved by the Ethics Committee of the Affiliated Changsha Hospital of Hunan Normal University.
Figures




Similar articles
-
Harpagide from radix scrophulariae attenuated ischemic stroke injury through calcium homeostasis regulation between mitochondria-associated membranes via the IP3R1/GRP75/VDAC1 complex.J Ethnopharmacol. 2025 Jul 24;351:120147. doi: 10.1016/j.jep.2025.120147. Epub 2025 Jun 13. J Ethnopharmacol. 2025. PMID: 40517916
-
Resveratrol inhibits autophagy in cardiomyocytes subjected anoxia/reoxygenation injury: involved in VDAC1/PINK1/Parkin pathway.Toxicol Appl Pharmacol. 2025 Sep;502:117421. doi: 10.1016/j.taap.2025.117421. Epub 2025 May 29. Toxicol Appl Pharmacol. 2025. PMID: 40449754
-
Mir221- and Mir222-enriched adsc-exosomes mitigate PM exposure-exacerbated cardiac ischemia-reperfusion injury through the modulation of the BNIP3-MAP1LC3B-BBC3/PUMA pathway.Autophagy. 2025 Feb;21(2):374-393. doi: 10.1080/15548627.2024.2395799. Epub 2024 Sep 8. Autophagy. 2025. PMID: 39245438 Free PMC article.
-
Insight into myocardial ischemia-reperfusion injury from the perspective of ferroptosis.Perfusion. 2025 Jul;40(5):1088-1102. doi: 10.1177/02676591241280371. Epub 2024 Sep 12. Perfusion. 2025. PMID: 39264884 Review.
-
The Function of Circular RNAs in Myocardial Ischemia-Reperfusion Injury: Underlying Mechanisms and Therapeutic Advancement.Cardiovasc Drugs Ther. 2025 Aug;39(4):875-886. doi: 10.1007/s10557-024-07557-1. Epub 2024 Jan 30. Cardiovasc Drugs Ther. 2025. PMID: 38289452 Review.
References
-
- Thiara, B. Cardiovascular disease. Nurs. Stand.29 (33), 60. 10.7748/ns.29.33.60 (2015). s44. - PubMed
-
- Lu, L., Liu, M., Sun, R., Zheng, Y. & Zhang, P. Myocardial infarction: symptoms and treatments. Cell. BiochemBiophys. 72 (3), 865–867. 10.1007/s12013-015-0553-4 (2015). - PubMed
-
- Bugger, H. & Pfeil, K. Mitochondrial ROS in myocardial ischemia reperfusion and remodeling. BiochimBiophys Acta Mol. Basis Dis.1866 (7), 165768. 10.1016/j.bbadis.2020.165768 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

