Comparative analysis of humoral immunity kinetics following three COVID-19 vaccines in a multi-ethnic cohort of medical students and healthcare professionals across Malaysia
- PMID: 40595319
- PMCID: PMC12216813
- DOI: 10.1038/s41598-025-07895-6
Comparative analysis of humoral immunity kinetics following three COVID-19 vaccines in a multi-ethnic cohort of medical students and healthcare professionals across Malaysia
Abstract
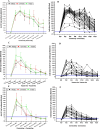
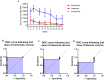
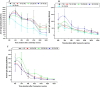
The objective of our study was to evaluate the time-dependent humoral immunity kinetics among multi-ethnic population of medical students and healthcare professionals across Malaysia following two doses of Comirnaty, Vaxzevria, and CoronaVac vaccines. Prospective observational cohort research was carried out from September 2021-March 2023. 242 vaccine recipients have completed the follow-up. After second vaccination dosage, peripheral blood was drawn every four weeks from week 0-24. Anti-S IgG and percentage of neutralization were measured from each individual plasma sample. Recipients of Comirnaty and Vaxzevria vaccine, exhibited adequate anti-S IgG and neutralizing antibody till week 24. In compared to Comirnaty/Vaxzevria vaccine group, CoronaVac group showed significant decrease of antibody as early as week 12. The Comirnaty vaccine peaked anti-S IgG production at week 4, while the Vaxzevria and CoronaVac vaccines peaked at week 2. The Comirnaty vaccine (91.3%) showed strong Ig-RBD neutralizing antibody response even after week 24, which led to the slowest rate of antibody waning when compared to CoronaVac (13%), which never achieves high Ig-RBD neutralizing antibody. The percentage of inhibition of Ig-RBD binding on ACE2 receptor and anti-S IgG seroconversion were significantly higher in the Comirnaty vaccine. The antibody response of Comirnaty was lower in recipients with higher BMIs than in those with normal or low BMIs. Higher BMI, however, was associated with more robust humoral immune responses to Vaxzevria vaccine. Following immunisation, the Chinese and Indian populations might exhibit a stronger humoral immune response than the Malay population.
Keywords: Comirnaty vaccine; Comparative antibody response; CoronaVac vaccine; Ethnicity; Malaysia; Time dependent response; Vaxzevria vaccine; Waning of antibody.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The human ethics committee of Manipal University College Malaysia had duly authorized the experimental design and techniques used in this investigation (MUCM/FOM/Research Ethics committee − 5/2021). Written informed consent was obtained from every participant before the inception of the study. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Safety comparison of mRNA, viral vector, and inactivated Covid-19 vaccines: incidence of adverse events following primary and booster doses among medical professionals in Malaysia.BMC Infect Dis. 2025 Jul 7;25(1):898. doi: 10.1186/s12879-025-11254-1. BMC Infect Dis. 2025. PMID: 40624462 Free PMC article.
-
A 22 month prospective assessment of neutralizing and IgG antibody levels against SARS-CoV-2 variants following homologous and heterologous BNT162b2 boosting.Sci Rep. 2025 Jul 1;15(1):21175. doi: 10.1038/s41598-025-05377-3. Sci Rep. 2025. PMID: 40594462 Free PMC article.
-
Immunogenicity and safety of CoronaVac vaccine in children and adolescents (Immunita-002, Brazil): A phase IV six-month follow up.Sci Rep. 2025 Jul 2;15(1):23040. doi: 10.1038/s41598-025-94596-9. Sci Rep. 2025. PMID: 40595400 Free PMC article. Clinical Trial.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
References
-
- First coronavirus cases in Malaysia: 3 Chinese nationals confirmed infected, quarantined in Sungai Buloh Hospital [Internet] Borneopost. ; (2020). Available from: https://www.theborneopost.com/2020/01/25/first-coronavirus-cases-in-mala...
-
- Worldometer, M. Corona virus cases and mortality. https://www.worldometers.info/coronavirus/country/malaysia/
-
- Malaysia confirms first cases of coronavirus infection | Reuters. Accessed December 6, (2022). Available at: https://www.reuters.com/article/china-health-malaysia/malaysia-confirms-...
-
- First Indian strain Covid-19 case detected in Malaysia. Accessed December 6, (2022). Available at: https://www.nst.com.my/news/nation/2021/05/687231/first-indian-strain-co...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

