Superior fulcrum reconstruction improve tendon-to-bone healing in irreparable massive rotator cuff tears compared with superior capsule reconstruction
- PMID: 40595372
- PMCID: PMC12215457
- DOI: 10.1038/s41598-025-09329-9
Superior fulcrum reconstruction improve tendon-to-bone healing in irreparable massive rotator cuff tears compared with superior capsule reconstruction
Abstract
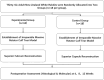
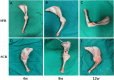
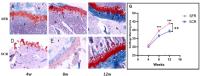
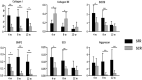
Recently, superior capsule reconstruction (SCR) has achieved some results in the treatment of irreparable massive rotator cuff tears (IMRCT), but the incidence of various postoperative complications is high. The study aims to establish a model of IMRCT in rabbits, and compare the histomorphology and molecular biology differences between superior fulcrum reconstruction (SFR) and SCR, so as to provide a new and effective treatment method for the clinical treatment of IMRCT. Thirty-six mature New Zealand white rabbits were required for the experiment to build the model of IMRCT. The supraspinatus and subscapular muscle of the thirty-six rabbits were cut off and randomly divided into two groups of eighteen rabbits in each group, with SFR and SCR. Six rabbits in each group were sacrificed at 4, 8, and 12 weeks after surgery for histological and molecular assessment. Macroscopically, no retear occurred in SFR group and SCR group after surgery. The results of molecular biological showed that the expression levels of COL1, BMP2, SCX and SOX9 in the SFR group were significantly higher than those in the SCR group at 4 and 8 weeks (P < 0.05), while the expression of COL3 was lower than that in the SCR group. There was no significant difference in the expression of Aggrecan between the two groups at 4 weeks (P > 0.05). At 12 weeks after surgery, the expression levels of COL1, BMP2 and Aggrecan in the SFR group were significantly higher than those in the SCR group (P < 0.05), while COL3, SCX and SOX9 were not significantly difference between the two groups (P > 0.05). Histologically, collagen fiber maturity and fibrocartilage regeneration in the SFR group were superior to those in the SCR group at 8 and 12 weeks (P < 0.05). However, at 4 weeks, there was no significant difference between the two groups (P > 0.05). In an IMRCT rabbit model, healing processes of SFR and SCR are different, but both repair techniques were effective. SFR outperformed SCR in collagen fiber maturity, fibrocartilage regeneration, and tendon regeneration.
Keywords: Histological; Irreparable massive rotator cuff tears; Molecularly; Rabbit model; Superior capsule reconstruction; Superior fulcrum reconstruction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: All the methods and experiments of this study were carried out in accordance with the relevant guidelines and regulations of Anhui Medical University. Moreover, the present animal study was performed in accordance with the guidelines and approval of the Institutional Animal Care and Use Committees (IACUCs) of Anhui Medical University. This study was conducted in accordance with the ethical standards set forth in the Declaration of Helsinki and its later amendments or comparable ethical standards. Moreover, our study is reported in accordance with ARRIVE guidelines. All procedures involving animal participants were approved by the Ethics Committee of The First People’s Hospital of Hefei (LLSC-YY2019-54).
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

