Autophagy controls differentiation of Drosophila blood cells by regulating Notch levels in response to nutrient availability
- PMID: 40595449
- PMCID: PMC12215389
- DOI: 10.1038/s41467-025-58389-y
Autophagy controls differentiation of Drosophila blood cells by regulating Notch levels in response to nutrient availability
Abstract
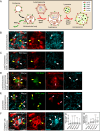
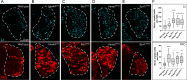
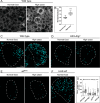
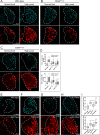
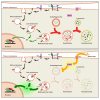
Drosophila larval hematopoiesis takes place at the lymph gland, where blood cell progenitors differentiate into two possible cell types: Plasmatocytes, analogous to mammalian macrophages, or crystal cells that share features with mammalian megakaryocytes; a third cell type, the lamellocytes, develop only upon specific immune challenges. Here we show that autophagy inhibition in blood cell progenitors results in augmented crystal cell differentiation due to Notch accumulation. Notch activation during hematopoiesis depends on the endocytic pathway, which crosstalks with autophagy: While Notch activation depends on endocytosis and endosomal maturation, Notch lysosomal degradation requires autophagy. TOR signaling inhibits autophagosome biogenesis that in turn prevents the formation of Notch-containing amphisomes, which are necessary for Notch lysosomal destruction. Reduction of Notch lysosomal degradation shifts the balance towards Notch activation at endosomal membranes, thereby enhancing differentiation of crystal cells. Our work therefore defines a mechanism of regulation of immune cell differentiation in response to the nutritional status of the organism.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

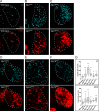
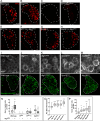
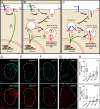
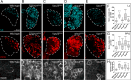
References
MeSH terms
Substances
Grants and funding
- PICT-2018-1501/Ministry of Science, Technology and Productive Innovation, Argentina | Agencia Nacional de Promoción Científica y Tecnológica (National Agency for Science and Technology, Argentina)
- PICT-2021-I-A-00240/Ministry of Science, Technology and Productive Innovation, Argentina | Agencia Nacional de Promoción Científica y Tecnológica (National Agency for Science and Technology, Argentina)
- PICT-2019-0621/Ministry of Science, Technology and Productive Innovation, Argentina | Agencia Nacional de Promoción Científica y Tecnológica (National Agency for Science and Technology, Argentina)
- PICT-2015-0225/Ministry of Science, Technology and Productive Innovation, Argentina | Agencia Nacional de Promoción Científica y Tecnológica (National Agency for Science and Technology, Argentina)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

