Reduction of neuronal activity mediated by blood-vessel regression in the adult brain
- PMID: 40595484
- PMCID: PMC12217672
- DOI: 10.1038/s41467-025-60308-0
Reduction of neuronal activity mediated by blood-vessel regression in the adult brain
Abstract
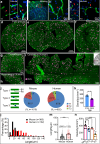
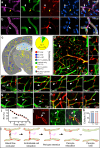
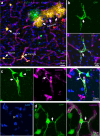
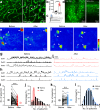
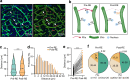
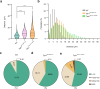
The brain vasculature supplies neurons with glucose and oxygen, but little is known about how vascular plasticity contributes to brain function. Using longitudinal in vivo imaging, we report that a substantial proportion of blood vessels in the adult mouse brain sporadically occlude and regress. Their regression proceeds through sequential stages of blood-flow occlusion, endothelial cell collapse, relocation or loss of pericytes, and retraction of glial endfeet. Regressing vessels are found to be widespread in mouse, monkey and human brains. We further reveal that blood vessel regression cause a reduction of neuronal activity due to a dysfunction in mitochondrial metabolism and glutamate production. Our results elucidate the mechanism of vessel regression and its role in neuronal function in the adult brain.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Zhang, Z. G. et al. Three-dimensional measurement of cerebral microvascular plasma perfusion, glial fibrillary acidic protein and microtubule associated protein-2 immunoreactivity after embolic stroke in rats: a double fluorescent labeled laser-scanning confocal microscopic study. Brain Res844, 55–66 (1999). - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

