Cell-specific mechanisms drive connectivity across the time course of Huntington's disease
- PMID: 40595490
- PMCID: PMC12218932
- DOI: 10.1038/s41467-025-60556-0
Cell-specific mechanisms drive connectivity across the time course of Huntington's disease
Abstract
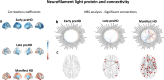
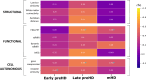
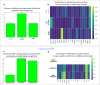
Hyperconnectivity in functional brain networks occurs decades before disease onset in Huntington's disease. However, the biological mechanisms remain unknown. We investigate connectivity in Huntington's disease using Morphometric INverse Divergence (MIND) in three Huntington's disease cohorts (N = 512) spanning from two decades before the onset of symptoms through to functional decline. Here, we identify stage-specific profiles, with hyperconnectivity 22 years from predicted motor onset, progressing to hypoconnectivity through the late premanifest and manifest stages, showing that hypoconnectivity is correlated with neurofilament light concentrations. To understand the biological mechanisms, we investigate associations with cortical organization principles including disease epicentres and cell-autonomous systems, in particular neurotransmitter distribution. The contribution from disease epicentres is limited to late premanifest while cell-autonomous associations are demonstrated across the Huntington's disease lifespan. Specific relationships to cholinergic and serotoninergic systems localized to granular and infragranular cortical layers are identified, consistent with serotoninergic layer 5a neuronal vulnerability previously identified in post-mortem brains.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

