TNF-α modulates cell proliferation via SOX4/TGF-β/Smad signaling in benign prostatic hyperplasia
- PMID: 40595502
- PMCID: PMC12217184
- DOI: 10.1038/s41419-025-07783-x
TNF-α modulates cell proliferation via SOX4/TGF-β/Smad signaling in benign prostatic hyperplasia
Abstract
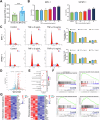
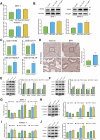
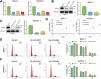
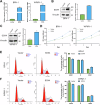
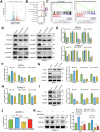
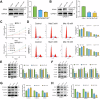
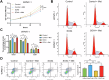
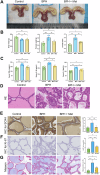
Benign prostatic hyperplasia (BPH) is an age-related condition in men with a poorly defined etiology. Chronic inflammation is increasingly recognized as a key contributor to BPH progression; however, the underlying mechanisms remain incompletely understood. This study aimed to elucidate the role of a TNF-α-induced inflammatory microenvironment in regulating BPH progression. We demonstrated that TNF-α levels were significantly elevated in patients with BPH and positively correlated with key clinical characteristics. In vitro, TNF-α promoted the proliferation of prostatic cells. Mechanistically, TNF-α induced the overexpression of SOX4, which subsequently activated the TGF-β/Smad2/3 signaling axis, thereby enhancing cellular proliferation, promoting epithelial-mesenchymal transition (EMT), and exacerbating fibrosis. Importantly, metformin (Met) treatment reduced the expression levels of relevant inflammatory cytokines in the serum of BPH rats. Further analysis confirmed that Met inhibited the TGF-β/Smad2/3 signaling pathway by downregulating the expression of SOX4, thus suppressing cell proliferation, reversing EMT, alleviating fibrosis, and ultimately exerting anti-BPH effects. Collectively, our findings suggest that TNF-α promotes BPH progression via activation of the SOX4/TGF-β/Smad2/3 axis, while Met exerts therapeutic effects by targeting this pathway. These results highlight SOX4 as a potential therapeutic target for BPH and support the clinical potential of Met in BPH management.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
References
-
- Devlin CM, Simms MS, Maitland NJ. Benign prostatic hyperplasia - what do we know? BJU Int. 2021;127:389–99. - PubMed
-
- Hoke GP, McWilliams GW. Epidemiology of benign prostatic hyperplasia and comorbidities in racial and ethnic minority populations. Am J Med. 2008;121:S3–10. - PubMed
-
- Vuichoud C, Loughlin KR. Benign prostatic hyperplasia: epidemiology, economics and evaluation. Can J Urol. 2015;22:1–6. - PubMed
-
- DerSarkissian M, Xiao Y, Duh MS, Lefebvre P, Swensen AR, Bell CF. Comparing clinical and economic outcomes associated with early initiation of combination therapy of an alpha blocker and dutasteride or finasteride in men with benign prostatic hyperplasia in the United States. J Manag Care Spec Pharm. 2016;22:1204–14. - PMC - PubMed
-
- Chang WH, Tsai YS, Wang JY, Chen HL, Yang WH, Lee CC. Sex hormones and oxidative stress mediated phthalate-induced effects in prostatic enlargement. Environ Int. 2019;126:184–92. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

