Aerobic and resistance exercise-regulated phosphoproteome and acetylproteome modifications in human skeletal muscle
- PMID: 40595512
- PMCID: PMC12216477
- DOI: 10.1038/s41467-025-60049-0
Aerobic and resistance exercise-regulated phosphoproteome and acetylproteome modifications in human skeletal muscle
Abstract
Despite indisputable benefits of different exercise modes, the molecular underpinnings of their divergent responses remain unclear. We investigate post-translational modifications in human skeletal muscle following 12 weeks of high-intensity aerobic interval or resistance exercise training. High-intensity aerobic training induces acetylproteome modifications including several mitochondrial proteins, indicating post-translational regulation of energetics machinery, whereas resistance exercise training regulates phosphoproteomic modifications of contractile/cytoskeletal machinery, consistent with greater strength. Furthermore, despite similar transcriptional responses to a single acute bout of aerobic and resistance exercise, more robust phosphoproteomic and metabolomic responses occur with acute aerobic exercise, including phosphorylation of structural/contractile and membrane transport machinery, and the nascent polypeptide-associated complex-α, a regulator of protein translation. Together, our findings provide new insight on the intricate phosphoproteomic and acetylproteomic modifications in muscle that potentially explain physiological responses to different modes of chronic and acute exercise. This study is registered with ClinicalTrials.gov, numbers NCT01477164 and NCT04158375.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: C.M.A. is a shareholder and serves as an officer and K.S.N. is on the advisory board at Emmyon, Inc. The remaining authors declare no competing interests.
Figures

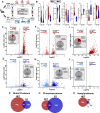



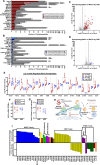

References
-
- Hickson, R., Bomze, H. & Holloszy, J. Linear increase in aerobic power induced by a strenuous program of endurance exercise. J. Appl. Physiol.42, 372–376 (1977). - PubMed
-
- Holloszy, J. O. Biochemical adaptations in muscle: effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem.242, 2278–2282 (1967). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- K01 DK141911/DK/NIDDK NIH HHS/United States
- K01DK141911/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- Robert and Arlene Kogod Center on Aging at Mayo Clinic: Innovation in Aging Award/Mayo Clinic | Center for Clinical and Translational Science, Mayo Clinic (CCaTS)
- R01 AG062859/AG/NIA NIH HHS/United States
- Dr. Emslander Professorship/Mayo Foundation for Medical Education and Research (Mayo Foundation)

