Reward history guides focal attention in whisker somatosensory cortex
- PMID: 40595521
- PMCID: PMC12219055
- DOI: 10.1038/s41467-025-60592-w
Reward history guides focal attention in whisker somatosensory cortex
Abstract
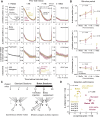
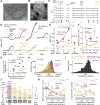
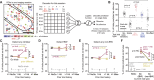
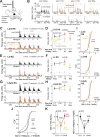
Prior reward is a potent cue for attentional capture, but the underlying neurobiology is largely unknown. In a whisker touch detection task, we show that mice flexibly shift attention between specific whiskers on a trial-by-trial timescale, guided by the recent history of stimulus-reward association. Two-photon calcium imaging and spike recordings reveal a robust neurobiological correlate of attention in the somatosensory cortex, boosting sensory responses to the attended whisker in L2/3 and L5, but not L4. Attentional boosting in L2/3 pyramidal cells is topographically precise and whisker-specific, and shifts receptive fields toward the attended whisker. L2/3 vasoactive intestinal peptide (VIP) interneurons are broadly activated by whisker stimuli, motion, and arousal but do not carry a whisker-specific attentional signal, and thus do not mediate spatially focused tactile attention. These findings provide an experimental model of focal attention in the mouse whisker tactile system, showing that the history of recent past stimuli and rewards dynamically engage local modulation in cortical sensory maps to guide flexible shifts in ongoing behavior.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Update of
-
Reward history guides focal attention in whisker somatosensory cortex.bioRxiv [Preprint]. 2024 Sep 9:2024.07.17.603927. doi: 10.1101/2024.07.17.603927. bioRxiv. 2024. Update in: Nat Commun. 2025 Jul 1;16(1):5580. doi: 10.1038/s41467-025-60592-w. PMID: 39131281 Free PMC article. Updated. Preprint.
Similar articles
-
Reward history guides focal attention in whisker somatosensory cortex.bioRxiv [Preprint]. 2024 Sep 9:2024.07.17.603927. doi: 10.1101/2024.07.17.603927. bioRxiv. 2024. Update in: Nat Commun. 2025 Jul 1;16(1):5580. doi: 10.1038/s41467-025-60592-w. PMID: 39131281 Free PMC article. Updated. Preprint.
-
Repetitive Sensory Stimulation Potentiates and Recruits Sensory-Evoked Cortical Population Activity.J Neurosci. 2025 Jan 22;45(4):e2189232024. doi: 10.1523/JNEUROSCI.2189-23.2024. J Neurosci. 2025. PMID: 39510832 Free PMC article.
-
Presynaptic maturation of inhibitory connections onto vasoactive intestinal polypeptide-expressing GABAergic interneurons in the mouse barrel field.Pflugers Arch. 2025 Aug;477(8):1089-1101. doi: 10.1007/s00424-025-03101-8. Epub 2025 Jun 25. Pflugers Arch. 2025. PMID: 40560417 Free PMC article.
-
Psychological and educational interventions for subfertile men and women.Cochrane Database Syst Rev. 2016 Mar 31;3(3):CD011034. doi: 10.1002/14651858.CD011034.pub2. Cochrane Database Syst Rev. 2016. PMID: 27031818 Free PMC article.
-
Behavioral and Neural Evidence of the Rewarding Value of Exercise Behaviors: A Systematic Review.Sports Med. 2018 Jun;48(6):1389-1404. doi: 10.1007/s40279-018-0898-0. Sports Med. 2018. PMID: 29556981
References
-
- Gallace, A. & Spence, C. in In Touch with the Future: The Sense of Touch from Cognitive Neuroscience to Virtual Reality 0 (Oxford University Press, 2014).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

