An ex vivo uterine system captures implantation, embryogenesis, and trophoblast invasion via maternal-embryonic signaling
- PMID: 40595542
- PMCID: PMC12217791
- DOI: 10.1038/s41467-025-60610-x
An ex vivo uterine system captures implantation, embryogenesis, and trophoblast invasion via maternal-embryonic signaling
Abstract
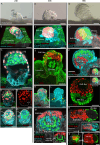
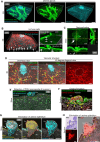
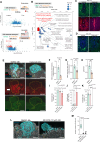
Embryo implantation remains challenging to study because of its inaccessibility in situ despite its essentiality and clinical significance. Although recent studies on long-term culture of authentic and model embryos have provided significant advances in elucidating embryogenesis in vitro, they, without the uterus, cannot genuinely replicate implantation. Here, we have recapitulated bona fide implantation ex vivo at more than 90% efficiency followed by embryogenesis and trophoblast invasion using authentic mouse embryos and uterine tissue. We utilized air-liquid interface culture method with originally developed devices manufactured with polydimethylsiloxane. Notably, the system replicated the robust induction of a maternal implantation regulator COX-2 at the attachment interface, which was accompanied by trophoblastic AKT activation, suggesting a possible signaling that mediates maternal COX-2 and embryonic AKT1 that accelerates implantation. By expanding the ex vivo findings, embryonic AKT1 transduction ameliorated defective implantation of uterine origin by a COX-2 inhibitor in vivo. The system, proposing a potentially standard platform of embryogenesis, offers a concise, reproducible, and scalable screening system, suggesting significant implications for developmental biology and therapeutic strategies for recurrent implantation failure in assisted reproductive technology.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Glujovsky, D. et al. Cleavage-stage versus blastocyst-stage embryo transfer in assisted reproductive technology. Cochrane Database Syst. Rev.5, CD002118 (2022). - PubMed
-
- Quenby, S. et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet397, 1658–1667 (2021). - PubMed
-
- Shapiro, B. S. et al. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil. Steril.96, 344–348 (2011). - PubMed
-
- Ohtani, T. et al. Dissection of signaling cascades through gp130 in vivo: reciprocal roles for STAT3- and SHP2-mediated signals in immune responses. Immunity12, 95–105 (2000). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

