Intraoperative nerve-specific fluorescence visualization in head and neck surgery: a Phase 1 trial
- PMID: 40595556
- PMCID: PMC12219357
- DOI: 10.1038/s41467-025-60737-x
Intraoperative nerve-specific fluorescence visualization in head and neck surgery: a Phase 1 trial
Abstract
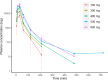
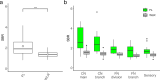
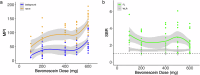
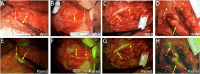
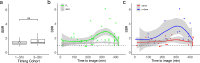
Iatrogenic nerve injury is a surgical complication with significant morbidity. This clinical trial, now complete, investigates the systemic administration of bevonescein, which selectively binds to nerves, in a single-arm, prospective multi-center, dose-escalation Phase 1 trial in adult patients with head and neck neoplasms undergoing parotidectomy or thyroidectomy in the United States. Twenty-seven participants are enrolled in the trial and receive the systemic agent. The primary outcome is safety with no dose-limiting toxicity among the 27 patients, but a single adverse event was identified that was possibly related to the study drug (vomiting). Secondary outcomes include the pharmacokinetics, optimal dose, and timing of bevonescein. The half-life of bevonescein is 29-72 min, and the optimal dose is 500 mg by objective measures, with the fluorescence signal-to-background ratio (SBR; 2.1 ± 0.8) significantly higher compared to white light (1.3 ± 0.2; p = 0.003). The fluorescent SBR of nerves between the early (1-3 h) versus late (3-5 h) timing cohorts is not statistically different. Here, we present data of a nerve imaging agent showing that preoperative intravenous infusion of bevonescein is well tolerated. This trial is registered at ClinicalTrials.gov (NCT04420689) and is sponsored by Alume Biosciences (San Diego, CA).
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Q.T.N. is a co-founder and interim-CEO of Alume. B.J.B. is a co-founder and Chief Medical Officer of Alume. A patent for the technology described in this manuscript has been filed with the United States Patent and Trademark Office (USPTO) by The Regents of the University of California (Q.T.N. and B.J.B.). The other authors declare no competing interests.
Figures
Similar articles
-
Safety and tolerability of intravenous liposomal GM1 in patients with Parkinson disease: A single-center open-label clinical phase I trial (NEON trial).PLoS Med. 2025 May 13;22(5):e1004472. doi: 10.1371/journal.pmed.1004472. eCollection 2025 May. PLoS Med. 2025. PMID: 40359409 Free PMC article. Clinical Trial.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Claudin 18.2-targeting antibody-drug conjugate CMG901 in patients with advanced gastric or gastro-oesophageal junction cancer (KYM901): a multicentre, open-label, single-arm, phase 1 trial.Lancet Oncol. 2025 Feb;26(2):227-238. doi: 10.1016/S1470-2045(24)00636-3. Epub 2025 Jan 6. Lancet Oncol. 2025. PMID: 39788133 Clinical Trial.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- Lee, Y. J. et al. Intraoperative fluorescence-guided surgery in head and neck squamous cell carcinoma. Laryngoscope131, 529–534 (2021). - PubMed
-
- Stibbe, J. A. et al. First-in-patient study of OTL78 for intraoperative fluorescence imaging of prostate-specific membrane antigen-positive prostate cancer: a single-arm, phase 2a, feasibility trial. Lancet Oncol.24, 457–467 (2023). - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

